Highlights
- Prescient ended the June quarter with a strong financial position and crossed major operating milestones
- During the reporting period, PTX unveiled CellPryme-M, an advanced CAR-T cell manufacturing enhancement technology
- Prescient has made steady progress in its OmniCar programs including in-vivo studies
Clinical-stage oncology company Prescient Therapeutics Limited (ASX:PTX) has provided an upbeat update for the June 2022 quarter, which saw ‘solid progress on all fronts’. The ASX-listed biotech firm, which is engaged in developing personalised therapies for cancer treatment, ended the quarter on a strong financial footing and well-advancing cancer programs. The company has its eyes on several value-creating milestones as it forged ahead with its business.
On that note, let us look at Prescient’s action-packed June quarter performance.
Finances
Prescient reported a cash balance of AU$12.3 million at the end of the quarter.
Costs for the quarter included:
- Ongoing clinical trials
- Manufacturing for PTX-100, PTX-200
- OmniCAR next-generation CAR-T platform and cell therapy manufacturing technology CellPrymeM
Total cash outflows
PTX reported total cash outflows of AU$1.2 million in the June quarter, with $0.5 million invested in research and development activities in Australia and the United States. Prescient’s management closely tracks the operating costs and the cash reserve management to enable the business to continue multiple programs.
Milestones during the June quarter 2022
Prescient, a clinical-stage cell therapy company developing targeted and cellular therapies for cancer treatment, is developing OmniCAR programs for next-generation CAR-T therapies. The company's major target indications are Acute Myeloid Leukemia (AML), Her2+ solid tumours, including breast, ovarian, gastric cancers, and glioblastoma multiforme (GBM).
The infographic below depicts major clinical progress made by Prescient in the June quarter:
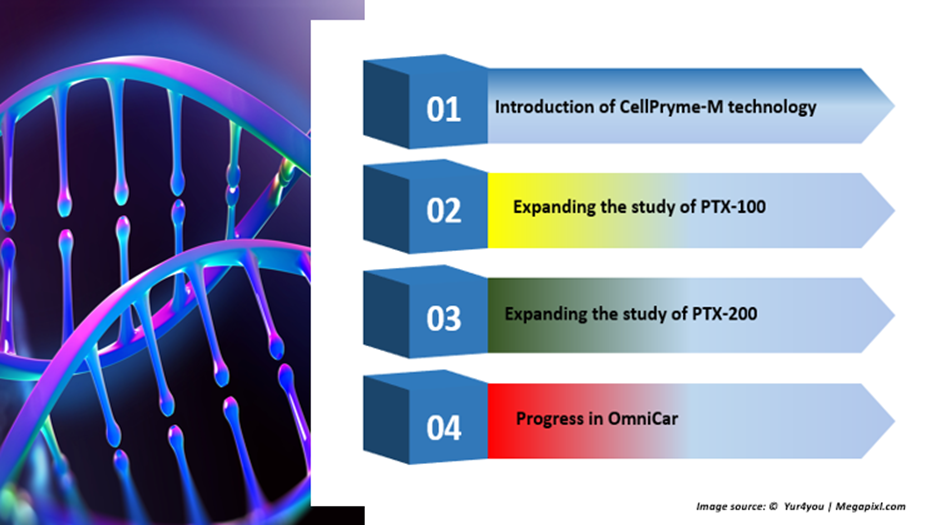
Image source: © 2022 Kalkine Media®
CellPryme-M technology
One of the company's key milestones during the period was the rollout of CellPryme-M. It is an advanced CAR-T cell manufacturing enhancement technology, which complements Prescient's OmniCAR platform.
CellPryme-M produces superior cells that can perform cancer-killing activity for a longer duration. It also holds the potential for improved tumour trafficking and penetrance compared to the existing generation of CAR-T cells. Overall, CellPryme-M CAR-T cells perform significantly better than the conventional CAR-T cells in highly aggressive solid cancer models.
CellPryme-M was developed in collaboration with PTX's research partner Peter MacCallum Cancer Centre. This technology has broad applications for improving Prescient's internal OmniCAR programs and current generation cell therapies. It paves the way for real commercial prospects for the company to integrate CellPryme-M into third-party production processes.
CellPryme-M is ready for clinical testing, with Good Manufacturing Practices (GMP) materials available to partners.
Expanding the study of PTX-100 in T-cell lymphoma patients
In an expansion cohort guided by Professor Miles Prince AM, Prescient is steadily enrolling patients with T-cell lymphomas (TCL). The company anticipates the completion of the recruitment by the end of the calendar year 2022.
After the June quarter, PTX-100 in July was granted Orphan Drug Designation by the US Food and Drug Administration (FDA) for treating peripheral T-cell lymphomas (PTCL).
Expansion of study in PTX-200
Another patient has shown complete remission of disease in a Phase 1b study of PTX-200 combined with chemotherapy (cytarabine). The study involved patients with relapsed and refractory acute myeloid leukemia (AML) at a dose of 45mg/m2. Previously, an additional partial response was reported, and till now, four patients have reported complete remission of their disease.
Progress in OmniCAR
Prescient has made steady progress in its OmniCAR programs, including in-vivo studies. Though it entails necessary optimisations in therapeutic development, the unfolding data confirms Prescient’s belief that OmniCAR is a transformative platform that can generate differentiated cell therapies.
Bottom line
Prescient and its collaborators are actively working towards delivering meaningful and substantial enhancements in the treatment of hard-to-treat cancers. The company’s strong cash balance of AU$12.3 million bodes well for executing multiple programs.
To know more about Prescient Therapeutics Limited, click here. Also, to stay updated with PTX company activities and announcements, please update your details on their investor centre.






