Highlights
- Prescient has launched its second cell therapy platform, CellPryme-M, which produces superior cells with greater cancer killing activity
- CellPryme-M, whose IP ownership is retained by Prescient, opens commercial opportunities for the company to incorporate it into third-party manufacturing processes
- CellPryme-M CAR-T cells show significantly better performance compared to conventional CAR-T cells in highly aggressive solid cancer models
- Users can incorporate CellPryme-M into their manufacturing process with virtually no interruption, even if their manufacturing timeframes are only 24-48 hours
In an upbeat development, clinical-stage oncology company Prescient Therapeutics (ASX:PTX) has rolled out a second cell therapy platform, “CellPryme-M”. It is a high-performance cell therapy manufacturing enhancement platform technology.
PTX developed the technology in association with its research partner, Peter MacCallum Cancer Centre (Peter Mac), a global cancer research leader.
Prescient retains the whole ownership of the intellectual property of CellPryme-M. This newly added technology platform opens new business prospects and commercial opportunities for Prescient to incorporate CellPryme-M into third-party manufacturing processes.
Why CellPryme-M is better than traditional CAR-T cells
During the cell production process, CellPryme-M produces superior cells, which are less susceptible to exhaustion. These cells can perform cancer killing activity for a longer duration and hold the potential for improved tumour trafficking and penetrance compared to the existing generation of CAR-T cells.
Overall, the CellPryme-M CAR-T cells show significantly better performance than conventional CAR-T cells in highly aggressive solid cancer models. It truly complements the OmniCAR platform, which enables control, multi-valency and many other characteristics and is agnostic to cell type.
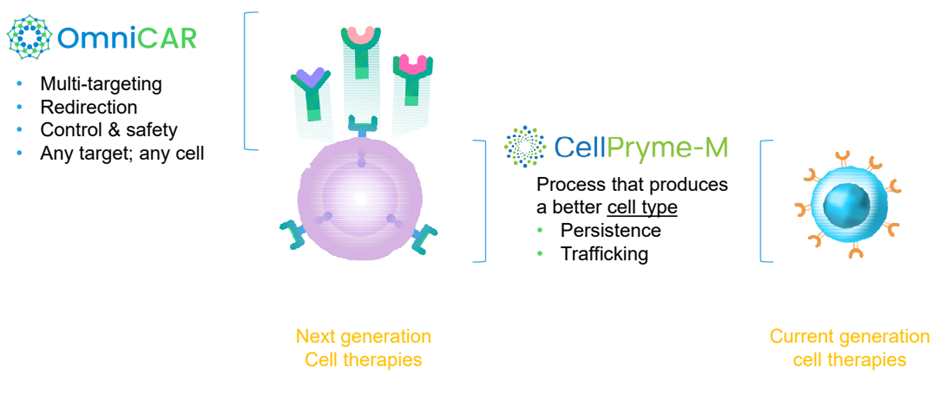
Image source: PTX investor presentation
Do read: Prescient (ASX:PTX) expands its PTX-200 trial in AML patients
Cells with more desirable phenotype
CellPryme-M produces cells with a superior phenotype, which increases the effectiveness of CAR-T therapy. It consists of a single, prompt step that can be adapted to standard cell manufacturing procedures. It works by influencing gene expression in immune cells resulting in:
- Down-regulation of genes linked with cell metabolism and protein folding
- Up-regulation of genes associated with interferon and cytokine signalling and genomic stability
Superior CAR-T cell phenotypes with greater proliferative capacity and long-lasting tumour killing activity are highly desirable as cell phenotype determines clinical anti-cancer responses. It is possible to control T-cell phenotype during the manufacturing process of cells. CellPryme-M produces these cells by altering the phenotype of T cells to a less differentiated state.
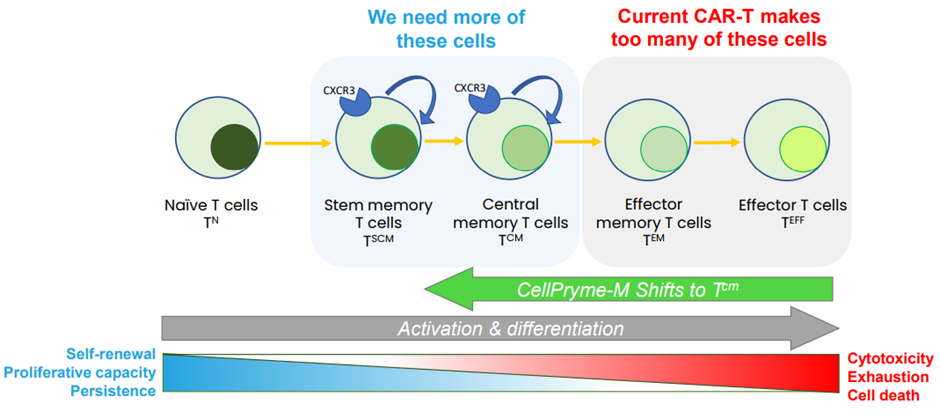
Image source: PTX investor presentation
The features of cells produced by CellPryme-M are as follows:
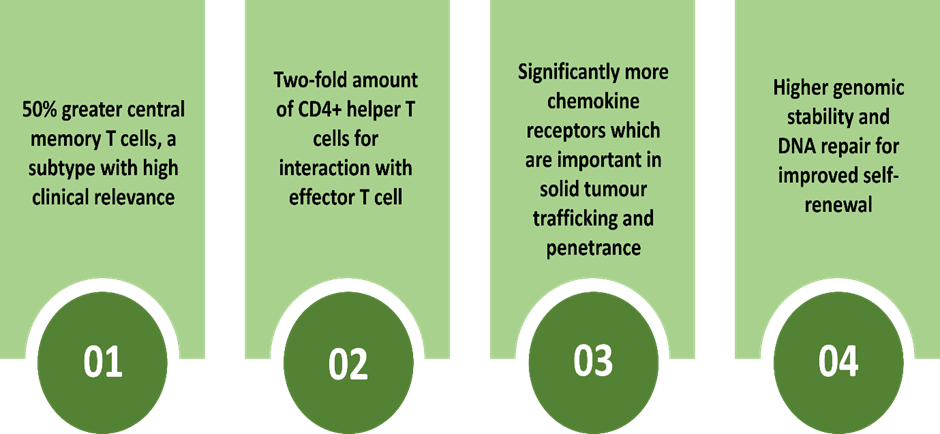
Image source: © 2022 Kalkine Media®
According to Prescient’s Senior Vice President of Scientific Affairs, Dr Rebecca Lim, CellPryme-M alters key signalling pathways within as little as 15 minutes, which means that users can incorporate CellPryme-M into their manufacturing process with virtually no interruption, even if their manufacturing timeframes are only 24-48 hours.
Doubled tumour control - The superior phenotype of CellPryme-M cells does not reduce the effectiveness of T cells, and they retain their potency with no safety risks due to higher cytokine release. Further, these cells exhibited almost doubled tumour control compared to conventional CAR-T and considerably improved survival in a mouse model of highly aggressive breast cancer, hugely resistant to conventional CAR-T.
Antiviral activity - During the development of CellPryme-M, there was rapid activation of genes relating to potent anti-viral pathways. This is an important finding as T cells with improved anti-viral activity might offer a definite gain in creating effective anti-viral therapies.
The road ahead
With exciting pre-clinical data, CellPryme-M is now ready for clinical testing. PTX plans to utilise CellPryme-M for enriching the cells used in its innovative OmniCAR programs. Further, PTX will pursue licensing of CellPryme-M to other cell therapy companies. This will improve conventional CAR-T programs and create value-adding alliances with external parties.
At the time of writing this article (WED 08 JUN 11:59 AM AEST), shares of PTX were trading at AU$0.215.
Also read: Cell therapies progress and strong cash position mark Prescient’s March quarter
To know more about Prescient Therapeutics Limited, click here. Also, to stay updated with PTX company activities and announcements, please update your details on their investor centre.





_04_24_2025_07_12_41_779131.png)