Highlights
- The March quarter saw Prescient make significant progress on the OmniCAR platform with pre-clinical work focused on different programs
- The expansion cohort in T-cell lymphomas started screening patients during the quarter.
- PTX made remarkable progress in its Cell Therapy Enhancements (CTE) program in collaboration with Peter MacCallum Cancer Centre.
Prescient Therapeutics (ASX:PTX) signed off the March quarter with major progress made in its Cell Therapy Enhancements and OmniCAR program.
The clinical-stage oncology company made inroads into multiple programs in its diverse and differentiated cancer portfolio.
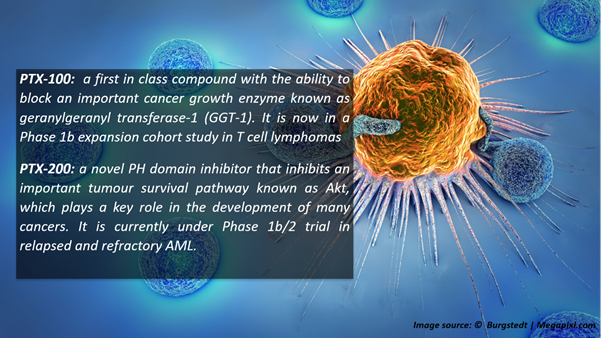
Currently, PTX is developing OmniCAR programs for next-generation CAR-T therapies, targeting several types of cancer. The company’s other targeted therapies include PTX-100 and PTX-200.
Related read: Prescient Therapeutics’ (ASX:PTX) 1H22 marked by key clinical milestones
Let us have a look at the developments made by the company during the March quarter.
OmniCAR Platform sees solid progress
During the quarter, Prescient made significant progress on the OmniCAR platform with pre-clinical work focused on three programs. These three indications are highly differentiated and use OmniCAR’s distinctive capabilities to create CAR-T therapies to solve the limitations faced by other CAR-T therapies.
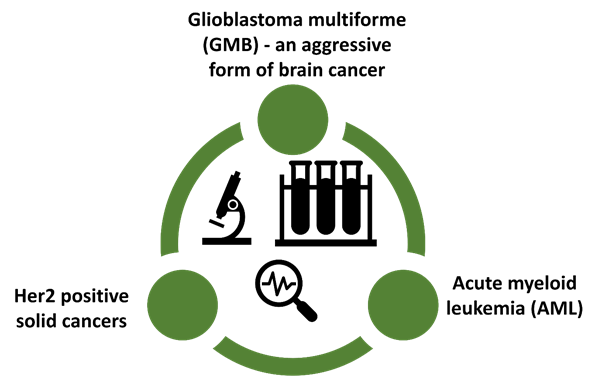
Image source: © 2022 Kalkine Media®
OmniCAR has the potential to deal with the limitations of current generation CAR-T. The platform is based on a technology licensed from Penn State University, Oxford University, and other assets.
What makes the OmniCAR platform unique?
- It has an ability to simultaneously target different cancer antigens
- It can be controlled post infusion
- It can accommodate any cancer targeting binder (to target a variety of cancers), and any immune cell type
Recruitment of patients for PTX-100 and PTX-200
Following the delivery of newly manufactured drug product from the US, the expansion cohort in T-cell lymphomas started screening patients. It was carried out under the leadership of Australia’s principal haematologist, Professor Miles Prince AM.
The expanded cohort will enrol throughout the year, with an interim readout possible in the coming quarters. Medical researchers are of the view that further favourable responses in this patient population could enable a subsequent registration study and a considerably shorter timeline to market, helping a patient population with essentially no present alternatives.
During the same period, the dose escalation study for PTX-200 was continued in patients with acute myeloid leukemia. To date, three patients enrolled in the study have shown complete responses.
Related read: Prescient Therapeutics (ASX:PTX) starts enrolling patients for extended PTX-100 trial for lymphomas
Partnership with the Peter MacCallum Cancer Centre
In collaboration with the globally reputed Peter MacCallum Cancer Centre, PTX is carrying out its Cell Therapy Enhancements (CTE) program, which aims to enhance the effectiveness and ability of the current generation of CAR-T treatments.

Image source: © 2022 Kalkine Media®
Strong cash position
Prescient reported a cash balance of AU$13.12 million at the end of the quarter. It made an investment worth AU$1.07 million in research and development, with a total cash outflow of AU$1.63 million.
Also read: Prescient Therapeutics (ASX:PTX) tables impressive results for December quarter
To know more about Prescient Therapeutics Limited, click here. Also, to stay updated with PTX company activities and announcements, please update your details on their investor centre.






