Highlights
- PTX-100 Phase 1b trial in r/r TCL has been completed with encouraging results.
- Phase 2 registration trial is under planning phase.
- PTX-100 has been granted US Orphan Drug designation by US FDA in all TCLs.
PTX-100 of Prescient Therapeutics Limited (ASX: PTX) is in spotlight as it is entering a new stage. The company highlighted that PTX is on the verge of a major inflexion point with the beginning of a PTX-100 Phase 2 study.
According to the company, PTX-100 has the ability to block a cancer growth enzyme called geranylgeranyl transferase-1 (GGT-1). It disrupts the Ras pathway by inhibiting the downstream activation of Ral, Rac, and Rho circuits in cancer cells, resulting in death of cancer cells.
Globally, PTX-100 is the only clinical stage RhoA (mutation recently identified) inhibitor.
Clinical status of PTX-100
PTX-100 Phase 1b trial aims to evaluate safety PK/PD. The study was conducted in patients with advanced malignancies, focusing on patients with refractory and relapsed T-cell lymphomas (r/r TCL). The trial showed excellent safety, target engagement at all three doses and preliminary efficacy in r/r TCL patients exceeding that anticipated from standard of care.
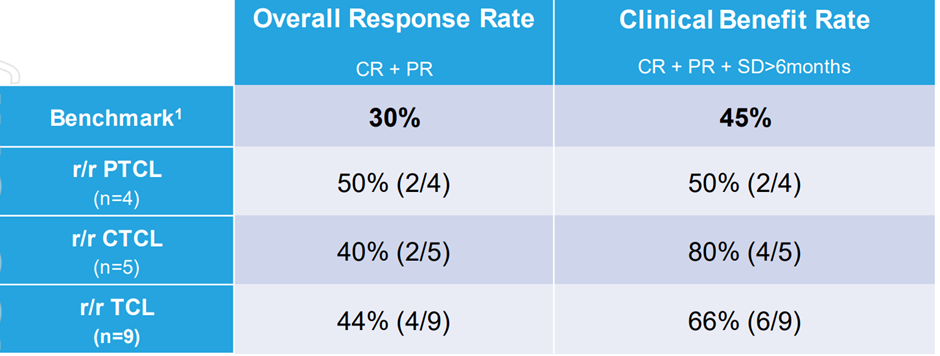
The trial demonstrated that PTX-100 has reported strong response rate in difficult diseases. The table below highlights the response rates –
PTX-100 has been granted US Orphan Drug designation in all TCLs by US FDA, which protects it for seven years post approval.
What’s ahead
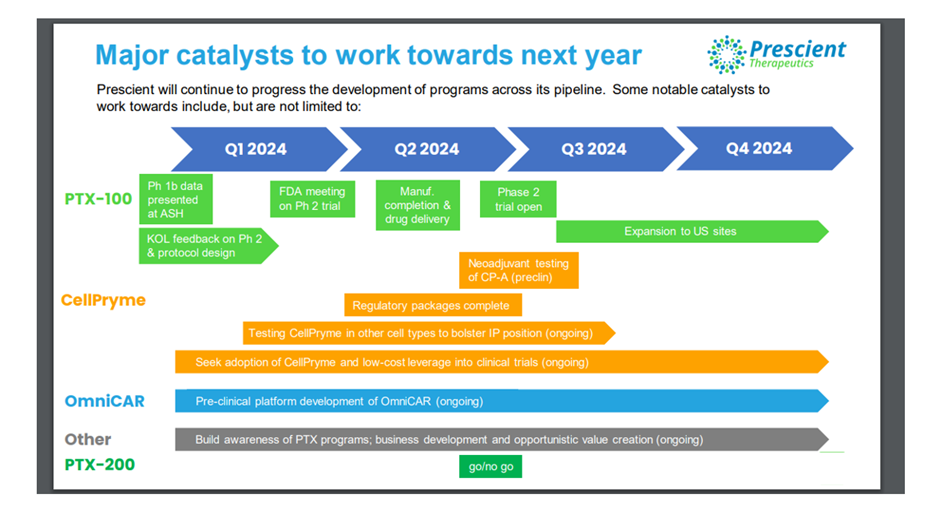
After finalising Phase 1 study, the company intends to undertake Phase 2 study in r/r TCL. For this purpose, it has engaged global KOLs (key opinion leaders) and regulatory consultants to design the study. The aim is to open study around mid 2024.
A manufacturing campaign is in progress to supply this study. Finalisation of manufacturing and drug delivery is expected to happen in 2Q 2024. Moreover, protocol drafting, and quality systems and controls are underway.
The company informed that FDA meeting is expected in 1Q/2Q 2024.
PTX-100 Phase 2 study commencement, a ‘watershed’ moment for PTX
Steven Yatomi-Clarke, MD and CEO of PTX, commented that the team is working towards the Phase 2 study, which it believes will be a ‘watershed moment for the company’.
The Phase-2 study will be the biggest catalyst in the history of PTX and the culmination of years of work, according to the firm. The trial has the potential to be a registration study, which means that the study requires drug to get into the market. It can boost clinical development and can truncate the funds and time desired to approve PTX-100.
The company said that in 2024, PTX could be the only ASX-listed firm with a drug in a registration study.