Highlights
- PTX is advancing its diversified pipeline of cancer therapies, backed by a world-class research, clinical and commercial team.
- PTX-100 Phase 1b trial data is expected in December 2023.
- Pre-clinical data of CellPryme surpasses the existing cell therapy approaches.
- Encouraging results have been reported from collaboration with Thermo Fisher.
Prescient Therapeutics Limited (ASX: PTX) is an Australia-based clinical-stage oncology company, committed to developing personalised cancer treatment.
In the September 2023 quarter, the company advanced its mission with further developments. The company ended the quarter with cash reserves of AU$18.7 million.
PTX to present PTX-100 Phase 1b trial data
PTX-100 Phase 1b trial is being conducted in patients with relapsed and refractory T cell lymphomas. So far, the trial has registered encouraging results.
The trial is nearing completion, and the company expects to share the results in December 2023.
Moreover, the company is set to attend a prestigious global haematology conference, presenting Phase 1b trial data.
In 1Q 2024, the company intends to meet the US FDA with focus on the design of Phase 2 trial, including the potential to use the trial as a registrational study and the endpoints. This development (trial as a registrational study) could create a faster and much shorter pathway to commercialisation and approval, if clinical trials are successful.
To assist the Phase 2 trial, the company has commenced the production of additional PTX-100 drug product.
PTX-200 trial nears completion
The PTX-200 study in acute myeloid leukemia (AML) is nearing completion. To finalise the data package, PTX seeks to enrol a final patient and assess any avenues for PTX-100 development.
CellPryme-M better than existing cytokines
Latest developments on CellPryme were presented at the ISCT-ANZ Regional Scientific Meeting in Perth.

Data source: Company update
The latest results indicated that pre-treatment with CellPryme has the capability to enhance the in vivo function of CAR-T cells expanded in IL2/7. The results are superior to existing cytokines.
Moreover, the most efficacious outcomes were reported when CellPryme-A and CellPryme-M were used in combination.
Collaboration with Thermo Fisher Scientific delivers significant results
PTX and Thermo Fisher Scientific reported collaboration results in September, using non-viral engineering of CAR-T cells to develop an enclosed, GMP-complaint manufacturing process.
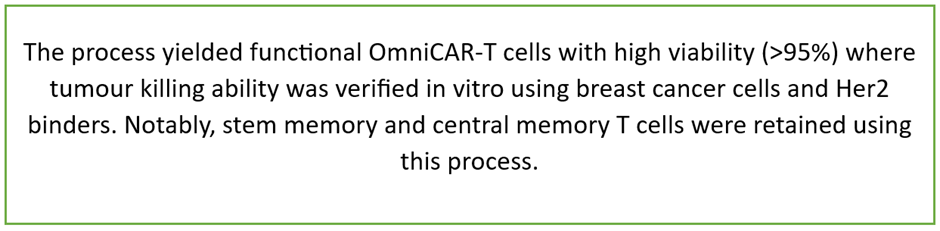
Data source: Company update
The company informed that the entire process is scalable on Thermo Fisher’s closed automated system for GMP manufacturing. This development is expected to improve the cost of goods for OmniCAR and enable decentralised manufacturing by third-party manufacturers.
In the coming quarters, the company intends to conclude the PTX-100 Phase 1b trial and hold a meeting with the FDA for design of the Phase 2 trial and initiation of the trial.
PTX shares traded at AU$0.072 on 21 November 2023.






