Medical device company Cortical Dynamics Limited which is headquartered in Australia remains focused on the development of Brain Anaesthesia Response Monitor (BARM) based on an industry disruptive technology. Cortical’s breakthrough system addresses a significant unmet need and helps in maintaining an optimum anaesthesia dose during major surgery. Shielded by five patent families with 22 registered patents across different jurisdiction around the world, BARM had already been granted both Therapeutic Goods Administration (TGA) approval and the CE mark.
Cortical’s BARM has a unique approach to calibrate anaesthetic monitoring to the individual patient rather than using a statistical average as used in systems developed by competitor products, resulting in improved patient outcome and saving time, money and lives.
Cortical’s fundamentally differentiated technology is focused on a pioneering technique of EEG monitoring involving a real-time measurement of a rhythmic electrical activity of patients’ brain which is documented via electroencephalogram (EEG).
A Robust Market Opportunity
The global brain monitoring market generated approximately $3,850 million in 2017. It is estimated that 312 million major surgical procedures around the world would require anaesthesia on an annual basis and 327,689 operating theatres globally.
In terms of single patient-use sensors to map brain activity, it is estimated that within USA, Western Europe and Australia there would be six million, five million and 188,000 of 24-hour single patient use sensors for imaging brain activity per annum respectively, comprising a total market opportunity of 11.188 million 24-hour single patient use sensors annually. With an average cost of A$20/single patient use sensor, an overall revenue stream is estimated to the tune of A$223.8 million/annum
Bearing in mind these statistics, Cortical’s Core product, BARM has huge potential to scale up. As it addresses two important patient monitoring market segments offering BARM instant expansion potential beyond the delivery of anaesthetic to brain monitoring in ICU, concussion and other areas. These segments are-
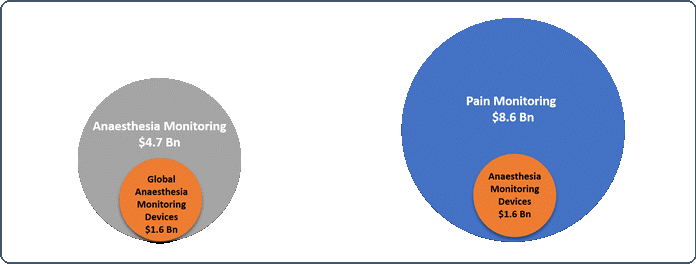
- Pain monitoring devices- The pain monitoring devices market is estimated to reach USD 8.6 billion per annum by 2022a.
- Anaesthesia monitoring devices- It is projected that the global anaesthesia monitoring devices market to reach over USD 1.6 billion by 2020b.
Substantial Market Opportunity in Europe Alone
The initial strategy of Cortical for commercialisation of BARM within Europe is concentrated on the Total Intravenous Anaesthesia (TIVA) market. TIVA is a process of inducing as well as maintaining general anaesthesia intravenously without using any kind of inhalation agent.
In the European Union, 20 % of the major anaesthesia procedures (29 million) being carried out annually, are total intravenous anaesthesia using propofol, whereas 55% (Circa 16million) are balanced anaesthesia that uses a mixture of intravenous agents such as volatile gases and propofol, and this creates a considerable market opportunity in Europe alone for Cortical valued between $83 million to $229 million.
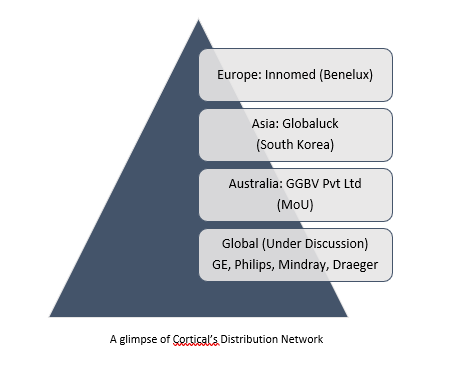
Cortical’s Distribution Channel
Cortical aims to strategically build a strong rapport with chief distributors in anticipation of boosting product sale and support.
- ANZ and Europe is Cortical’s immediate focus, where BARM is already being used. The first European Distribution agreement was signed by the Cortical in April 2018 wherein the Company collaborated with Innomed Benelux B.V. for the distribution of BARM in the Benelux countries: Belgium, Netherlands and Luxembourg. Distributor discussion is underway in UK, France and Germany.
- An exclusive 5 years distribution agreement with Globaluck in May 2018 to distribute BARM within South Korea, which was the first international agreement to be signed by Cortical. Globaluck has recently received the Koren Good Manufacturing Practice (KGMP) certificate of approval for BARM, a class II medical device after an independent Korean audit of Cortical’s facility in Scoreby, Victoria was conducted earlier in July
- For distribution in South Africa and other African countries an MoU has been signed with GGBV Pty Ltd in Australia.
- Cortical also foresees strong prospects to expand distribution into Japan, Taiwan and other countries.
- Cortical aims for integrated distribution of BARM with the top global brands in operation theatre monitoring equipment, GE, Philips, Mindray & Draeger.
A glimpse of Cortical’s Distribution Network
The accomplishment of TGA approval and the CE mark enabled “BARM” to be marketed and sold primarily into the United Kingdom, European, Asia-Pacific, South American markets.
Additional Opportunities
BARM’s versatility outranks the depth of anaesthesia which can be applied to a range of other EEG based markets, including drug discovery, neuro-diagnostic, drug evaluation, as well as the evolving Brain computer Interface (BCI) market.
There are other substantial opportunities offered by consequent extension of BARM by continuing development of the product in order to carry out additional functions, such as neuro-diagnostics of changes in brain and memory functions providing a timely warning of degenerative diseases, tranquiliser supervision & pain response for trauma patients in ICU’s.
Simply Put, with abundant market opportunities on its way, Cortical is well-positioned to move further up the ladder and progress BARM’s sale at a global scale expanding its footprints across Europe, Australia, New Zealand, Korea, Hong Kong and the USA.
- Global Brain Monitoring Devices Market Research Report”, accessed March 2017, #
- grandviewresearch.com/industry-analysis/pain-management-devices-market-April 2016
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. Kalkine Media does not in any way endorse or recommend individuals, products or services that may be discussed on this site. Our publications are NOT a solicitation or recommendation to buy, sell or hold. We are neither licensed nor qualified to provide investment advice.