Definition
Related Definitions
Medicines and Healthcare products Regulatory Agency (MHRA)
What is MHRA?
MHRA, or Medicines and Healthcare products Regulatory Agency, is an essential part of the United Kingdom’s Department of Health. Founded in 2003, the agency is responsible for regulating drugs, medical devices, along with blood elements for transfusions across the UK.
MHRA is involved in the protection and maintaining public health, supporting the development and innovation in this field. The agency manages both the CPRD (Clinical Practice Research Datalink) and the NIBSC (National Institute for Biological Standards and Control). The London-headquartered agency employs over 1,200 individuals across England.
Three main centres of the MHRA:
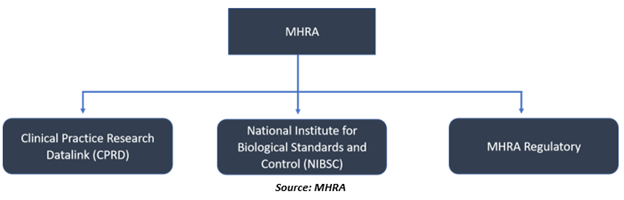
- CPRD provides a data research service that intends to make public health better by utilising NHS clinical information (anonymised).
- The NIBSC is engaged in the standardisation and management of biologics (biological medicines).
- MHRA is the regulator for the pharmaceutical and medical devices industries. Regulates medicines, medical devices, and blood components for transfusion, responsible for safeguarding their safety, quality, and efficacy in the UK.
Advisory bodies of the MHRA-
The MHRA has established several independent advisory committees for providing impartial advice to ministers related to the medicines and medical devices regulation. The committees may also create working groups to focus on specific challenges. The eight advisory bodies are as follows:

What does the MHRA do?
The MHRA ensures that any medicine or medical device is safe to use as well as meets the regulatory standards and required quality. A medical device could be a plaster, an inhaler for asthma, or a ventilator that is used in a hospital. All medicines require a licence from the agency before their prescription within the United Kingdom. This makes sure that the medicines are effective and safe to use.
The MHRA also monitors the application of new medicines. Doctors or patients can record any issues with the agency that might need further inquiry through the Yellow Card scheme.
The MHRA is accountable for the regulation of medical devices, equipment used in healthcare, medicines, along with the harmful incidents’ investigation. The agency regulates blood and blood-related products, working with UK based healthcare providers, blood services, and some other relevant organisations for improving blood quality and safety.
What are the roles & responsibilities of MHRA?
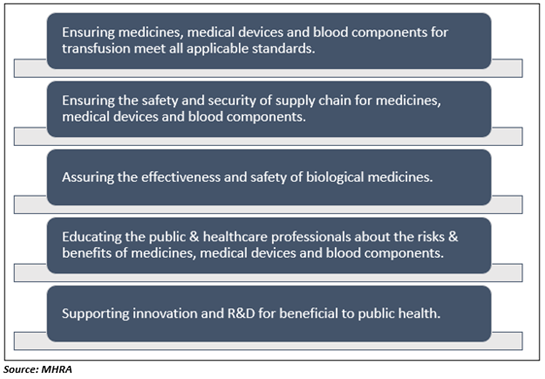
The key responsibilities of the MHRA are highlighted below:
- The agency makes sure that medical devices, medicines, and any blood components for transfusion meet the safety, quality, and efficacy applicable standards.
- MHRA ensures that the safety and security of the supply chain for medicines, medical devices, and blood components.
- The agency helps to promote international harmonisation & standardisation to ensure the safety and efficacy of biological medicines.
- MHRA helps to educate healthcare professionals (HCPs) and public about the risks and benefits of medical devices, medicines, and blood components. This way, the agency leads to safer and more efficient use.
- The agency provides support to the innovation, including the R&D that is performed for the benefit of public health.
Priorities of the MHRA
Some key focus areas for the Medicines and Healthcare products Regulatory Agency are-
- To make regulation more supportive of safe and reliable innovation.
- To support the growth agenda of the government through the life science strategy, which comprises the adaptive licensing pilot and early access to medicines schemes.
- To introduce a combined reporting system for adverse reactions, medical devices, medicines, blood & counterfeit products under the YC brand to make sure of the safety of the patient.
- To work with its partners across the United Kingdom, Europe and worldwide for preventing substandard therapeutic products entering into the supply chain.
- To establish a centre of excellence for advanced and innovative therapies, including stem cells.
- To engage more with patients, HCPs, and the public, including by working together with others in the healthcare system.
- To grow the volume of observational and interventional research, together with clinical trials, by using the Clinical Practice Research Datalink (CPRD) data.
What is the role of the MHRA in clinical studies?
Medicines and Healthcare products Regulatory Agency (MHRA) plays a vital role in the drug development process. For a clinical trial for a new drug to commence, it must first be assessed and authorised by the MHRA.
The agency examines sites where the clinical trials are conducted to ensure they are carried out in accordance with good clinical practice.
The clinical trials are designed to demonstrate the effect of new medicines as per the anticipations. The results from the clinical trials are sent to the MHRA. After the assessment of the clinical trial results, the agency decides whether to allow the sponsor or healthcare company manufacturing the medicine to market it for a particular indication.