Highlights
- Imugene Limited (ASX:IMU) has recently announced the publication of CHECKVacc abstract at ASCO 2022.
- The published abstract covers details about the Company’s Phase 1 CHECKVacc trial in adults with triple negative breast cancer.
- Imugene is addressing the unmet need of patients living with triple negative breast cancer through CHECKvacc.
Imugene Limited (ASX:IMU) has recently announced the publication of the CHECKVacc abstract at the 2022 Annual Meeting of the American Society of Clinical Oncology (ASCO). The abstract published at ASCO 2022 provides details about the Company’s Phase I CHECKVacc trial in adults with TNBC (triple negative breast cancer).
Imugene is tapping the huge breast cancer market through the oncolytic virus CHECKvacc, which is a novel chimeric orthopoxvirus with strong anti-cancer activity in TNBC xenografts. The Company expects CHECKvacc (CF33-hNIS-anti-PD-L1) to deliver an improved outcome for several women who are diagnosed with triple negative breast cancer each year.
Imugene Limited tapping breast cancer market via oncolytic virus CHECKvacc
CHECKvacc abstract
The published abstract is titled “Phase I study of intratumoral administration of CF33-HNIS-antiPDL1 in patients with metastatic triple negative breast cancer”. The lead author of the abstract is Dr Yuan Yuan MD, PhD from City of Hope National Medical Center, Duarte, California. He is also the Principal Investigator leading the Phase 1 CHECKVacc trial.
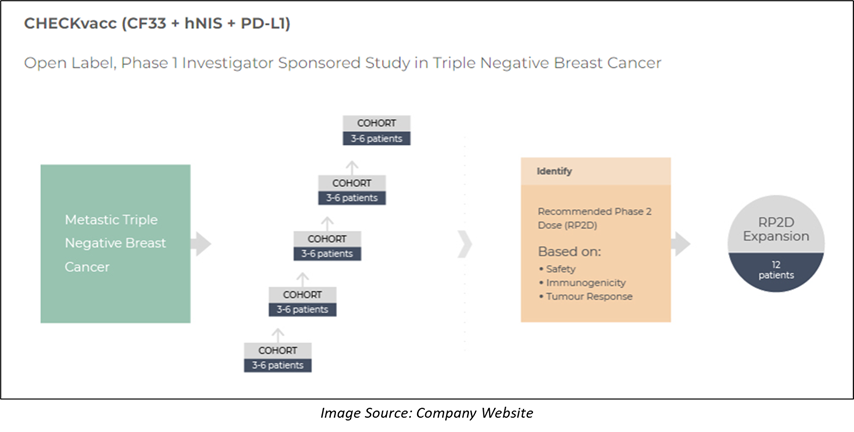
Imugene is progressing with the first-in-human, Phase I, single center, single arm clinical trial of CHECKvacc, assessing the safety and tolerability of CHECKvacc intratumoral injection in patients with metastatic TNBC. While the primary objective of the clinical trial is to evaluate the safety and tolerability of CHECKvacc, the secondary objectives are to determine the optimal biological dose, recommended Phase II dose, and the response rate.
Related Read: Imugene posts another achievement: PD1-Vaxx abstract published at ASCO 2022
Status of Phase I CHECKVacc trial
In April 2022, Imugene announced that City of Hope® has dosed the first cohort 2 patient in the Phase I CHECKvacc trial. The Company proceeded to the second dose cohort in March 2022 after the recommendation from the Protocol Management Team.
The Protocol Management Team agreed on CHECKvacc to be safe with no serious adverse reactions plus no dose-limiting toxicities seen following the review of the first low dose cohort of patient’s data. The existing trial design will include a dose escalation, followed by an expansion to around 12 patients at the final dose. The final dose will be the recommended phase II dose.
Imugene’s CHECKvacc is aimed at providing an improved clinical outcome of TNBC, which has limited treatment options. So far, there is no effective targeted therapy in patients with metastatic TNBC, except for tumours with a germline BRCA mutation. Meanwhile, breast cancer cases have been continuously rising in Australia, creating a significant market opportunity for the Company.
Imugene shares are trading at A$0.197 as of 12:52 PM AEST.



