Clinical-stage Oncology Company, Prescient Therapeutics Limited (ASX: PTX), that develops personalised medicine approaches to address mutations causing cancer. Prescientâs deep product pipeline include 2 clinical stage drugs with multiple shots on goal, PTX-100 and PTX-200, that turn off âmaster switchesâ known to drive cancer.
Encouraging Results Unveiling Complete Eradication of Cancer in Three Acute Myeloid Leukemia Patients
Prescient recently provided an update on encouraging results from its Phase 1b study with PTX-200 in patients with acute myeloid leukemia (AML), unveiling total eradication of disease in three of 15 patients in a notoriously difficult to treat cancer. Following the news on 27 November 2019, PTX shares soared by 100% amid heavy volumes, it finally settled the dayâs trade at $0.100, up by 33% and created excitement among the market enthusiasts.
PTX-200 in Relapse and Refractory Acute Myeloid Leukemia
The Phase 1b trial is being conducted at prestigious cancer institutes in the US; H. Lee Moffitt Cancer Center (Moffitt) situated in Florida, in association with University of Kansas Medical Center, Kansas and Yale Cancer Center in New Haven, Connecticut (Yale), led by world-renowned leukemia expert Professor Jeffrey Lancet.
In this study a total 15 relapsed, or refractory AML patients were being treated with Prescientâs novel drug PTX-200. The study showed that 3 of total 15 relapsed or refractory AML patients demonstrated complete responses, meaning 100 per cent eradication of cancer, in a hard to treat cancer population. These three patients received between 25-35 mg/m2 PTX-200, in combination with 200-400 mg/m2 chemotherapy agent cytarabine.
Expansion of AML study with amendment in protocol
Prescient is undergoing discussion with the study investigators for making a protocol amendment in order to modify the dosing schedule of PTX-200 regarding the administration of chemotherapy agent cytarabine dosage, with an aim to minimise overlapping drug interactions.
Prescient had reported successful outcomes in the previous Phase 1b trial in acute leukemias where PTX-200 was used as a monotherapy that resulted in -
- one CR, two PRs in r/r AML; one response in refractory CMML
- Overall, 53% SD in a highly pre-treated population diagnosed with advanced disease.
- A reduced p-Akt in AML patient blasts
The company further informed that no such side effects were seen in the previous Phase 1b study, indicating that the side effects observed in the present study could be a result of overlapping interaction of cytarabine and PTX-200.
It was further informed that in general, most patients undergoing treatment on the study by group have demonstrated tolerance to planned dose levels while an increase in transaminase was observed in three patients, though only one was dose limiting.
The revised protocol will go through the customary FDA and ethics committee reviews and the Company is expecting to re-start the enrollment in early 2020.
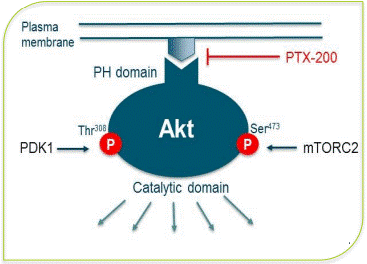
Novel Mode of action of PTX-200
PTX-200 (triciribine phosphate monohydrate) is an effective small molecule, positioned as a novel PH domain inhibitor that inhibits a vital tumor survival pathway known as Akt having a significant role in the progression of a range of cancers, including breast and ovarian cancer, as well as leukemia.
Other existing Akt inhibiting drug candidates are non-specific kinase inhibitors and have toxicity problems, PTX-200 differs from these drugs by incorporating its novel mode of action that exclusively inhibits Akt and being relatively safer while also conferring resistance to many therapies.
Source: Company Presentation
Prescientâs success is marked by the significant support received from the government as US FDA has granted PTX-200 an Orphan Drug Designation. The company explained that the occurrence of AML has risen to a significant level in the United States alone, however treatment has hardly been improved in last forty years, making it an area of high interest for investors and clinicians.
A Glimpse of Prescientâs Product Portfolio Involving PTX-200
Apart from Acute Myeloid Leukemia (AML), Prescient is also conducting several clinical trials in breast cancer, ovarian cancer for the highly promising compound PTX-200, evaluating it as a new therapy underpinned by convincing proof of concept and well-established safety profile.
Prescientâs PTX-200 Portfolio
In a nutshell, Prescient is progressing well with its robust product portfolio and currently undergoing several clinical trials foreseeing further promising results.
To know more about Prescient Therapeutics Limited, click here.
To stay updated with PTX company activities and announcements, please update your details on their investor centre.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. The above article is sponsored but NOT a solicitation or recommendation to buy, sell or hold the stock of the company (or companies) under discussion. We are neither licensed nor qualified to provide investment advice through this platform.


_07_04_2025_07_17_24_352003.jpg)