Highlights
- Radiopharm Theranostics is developing a world‐class platform of radiopharmaceutical and nuclear medicine products for both diagnostic and therapeutic uses.
- For the first half ended 31 December, the company has reported encouraging developments including new supply agreements, advanced clinical studies, and US FDA recognition.
- The company also boosted its financial position with a successful capital raising program.
- Subsequent to the period, the company entered a new agreement for acquiring a private US venture, tapped new expertise, and initial process for NASDAQ listing.
Radiopharm Theranostics Limited (ASX:RAD) saw significant developments in the half year period ended 31 December 2022. Some of the key developments were-- successful capital raising program, encouraging cancer study outcomes, new supply agreements, and critical approvals and drug status by the US FDA. Also, the company formed a new entity, Radiopharm Ventures, LLC, in a joint venture with The University of Texas MD Anderson Cancer Center.
The ASX-listed oncology space player continued the momentum into the second half of FY23 with the initiation of process for NASDAQ listing, new appointments, and a binding agreement to acquire private US-based venture Pharma15 Corporation.
The first-half highlights
New joint venture entity - Radiopharm Theranostics (USA), Inc. and The University of Texas MD Anderson Cancer Center announced the launch of Radiopharm Ventures, LLC. This new entity will focus on the development of novel radiopharmaceutical therapeutic products for cancer.
Encouraging study outcomes - The Pivalate Phase 2a data in patients with brain metastases indicated that F‐18 Pivalate PET showed high uptake regardless of the origin of the primary tumour.
The period saw the publication of positive imaging data regarding the HER2 nanobody (RAD201) in the prestigious European Journal of Nuclear Medicine & Molecular Imaging.

Data source: company update
Critical approvals and drug status by the US FDA - The US FDA gave a green signal to the company’s Investigational New Drug Application (IND) for αVβ6 Integrin (RAD301) technology. This development enables the company to kick off a Phase 1 imaging trial in patients with pancreatic cancer.
Also, the company secured Orphan Drug Designation for its DUNP19 technology from the US regulator. The drug is being studies in a rare bone cancer, osteosarcoma, which primarily affects adolescents, children, and young adults.
Another major milestone was the FDA grant of Rare Paediatric Disease (RPD) designation to the DUNP technology, which aims to treat serious, rare paediatric diseases.
Strategic partnerships - Under a collaboration agreement with Lantheus, the company is developing NM‐01, a nanobody for the potential diagnosis and treatment of multiple tumour types.
The company also extended its agreement with GenesisCare to support a second clinical trial in prostate cancer using its PSA targeting antibody.
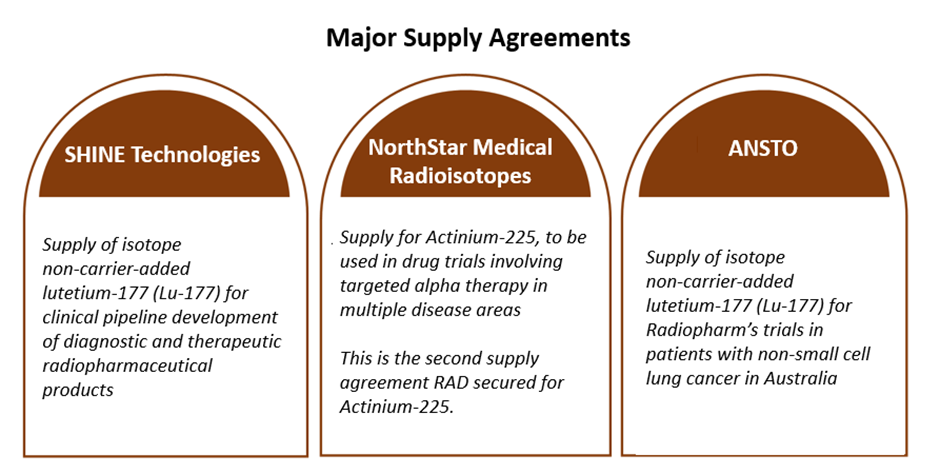
Data source: company update
Capital raising – The company closed an entitlement offer, raising AU$5.5 million through the institutional component and AU$1.2 million through the retail component. This capital raising program provides RAD with runway until at least the end of 2023, says the company.
New appointments, acquisition and NASDAQ listing
Subsequent to the first-half period, the company announced its intention to get listed on the NASDAQ, for which the process has been initiated.
Also, the company roped in Dr. Rama Abu Shmeis as Senior Vice President (SVP), Chemistry, Manufacturing, and Controls (CMC). Dr Shmeis holds the top shelf experience in the US market. To know more about this new senior team member at RAD, click here.
Most recently, the company entered into a new agreement to acquire Pharma15 Corporation.

Data source: company update
RAD shares were trading at AU$0.135 midday on 7 March 2023.




