Highlights
- PTX-100 has demonstrated an excellent safety profile in the Phase 1b expansion cohort study in relapsed and refractory T cell lymphomas (TCLs).
- PTX-100 continues to show encouraging clinical activity in the difficult-to-treat patient population.
- Prescient will recruit additional cutaneous TCL patients.
Clinical-stage oncology firm Prescient Therapeutics Limited (ASX:PTX), has provided an upbeat update regarding the encouraging clinical activity of PTX-100, one of the targeted therapies of Prescient. PTX-100 is a first-in-class compound with the potential to block an important cancer growth enzyme known as geranylgeranyl transferase-1.
PTX-100 demonstrated an excellent safety profile at the highest dose of 2,000 mg/m2 in a Phase 1b expansion cohort study in relapsed and refractory T cell lymphomas (TCLs) led by a globally renowned haematologist, Professor H. Miles Prince. The study was conducted at Epworth Hospital in Melbourne, Australia.
Additionally, PTX-100 continues to exhibit promising clinical activity in a difficult-to-treat patient population. It includes a striking response in a patient with refractory cutaneous TCL (CTCL).
Embed video: https://www.youtube.com/watch?v=9Fp_rY-qSRg
Patient enrolment
As of now, the expansion cohort has screened eight patients, out of which seven patients have been dosed with PTX-100 at 2,000 mg/m2. Of the seven, four had peripheral TCL (PTCL), and three had CTCL. Patients had received a median of four prior lines of therapy and up to six prior lines of therapy. Four patients remain on therapy and additional patients are being recruited.
PTX-100 has demonstrated an excellent safety profile in the study, with very few adverse events so far.
Clinical activity
The expansion cohort was aimed at evaluating safety; however, PTX-100 continued to exhibit encouraging clinical activity in the difficult-to-treat patient population. Below is a list of the observed responses:
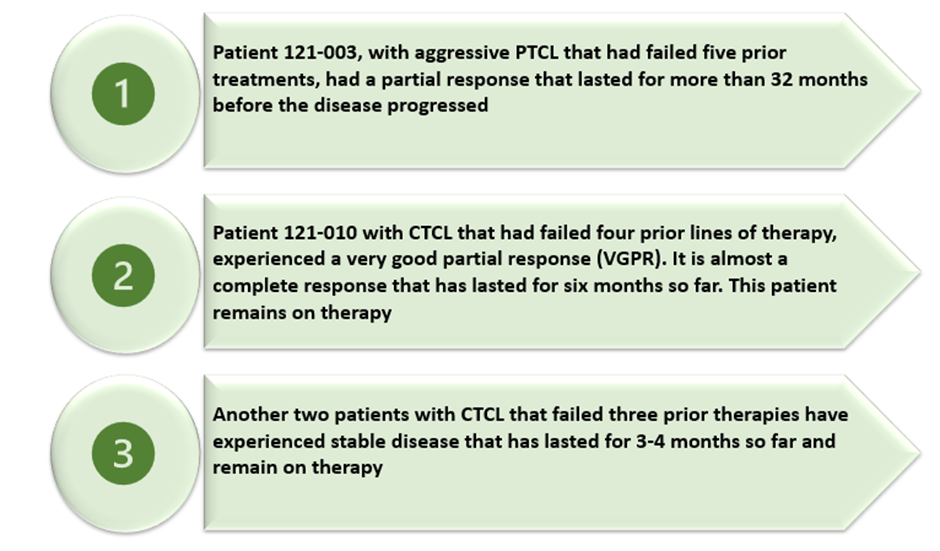
Data and image source: Company update
© 2022 Kalkine Media®
The road ahead
Given the encouraging responses, particularly in CTCL, Prescient has revised the study protocol to adjust the recruitment of additional CTCL patients. Recruitment remains on schedule, despite the new target of recruiting additional CTCL patients, which may correspondingly extend the study period. Prescient will continue the study while patients continue to derive clinical benefits from PTX-100.
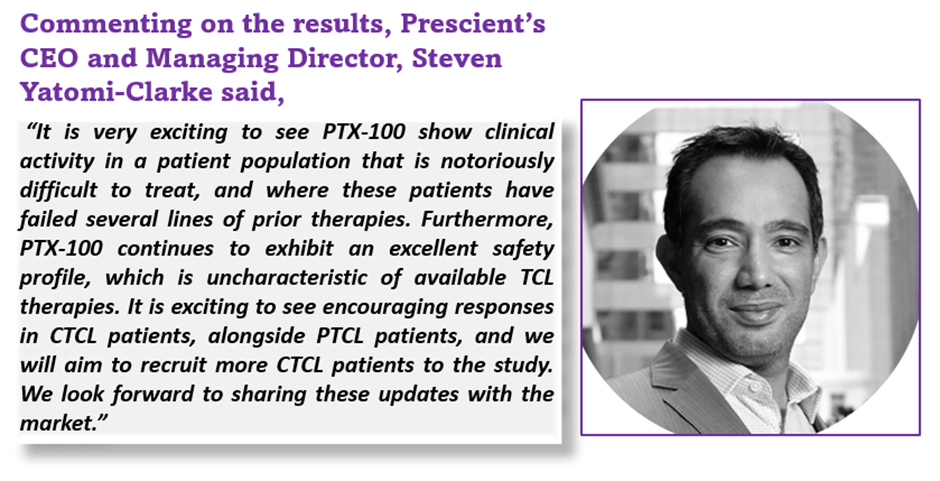
Data and image source: Company update
© 2022 Kalkine Media®
Stock information
PTX shares were noted at AU$0.165 (as on 26 October 2022)



