Highlights
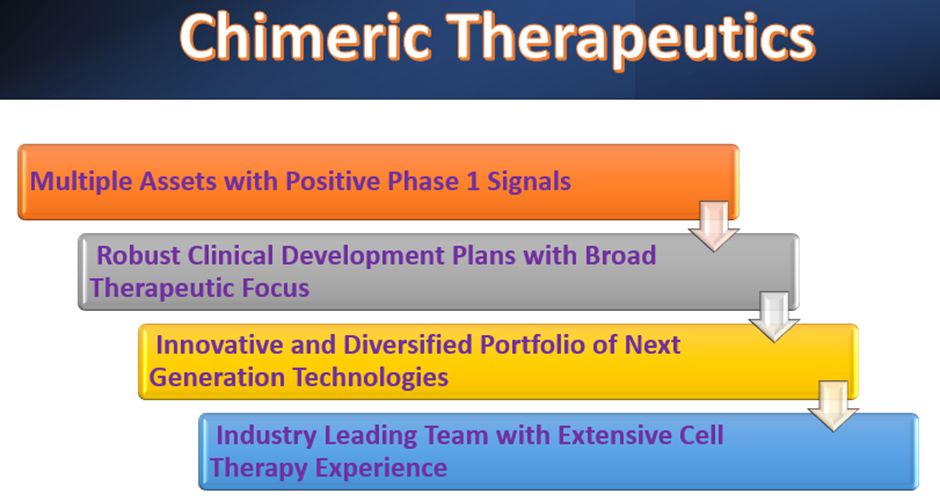
- Chimeric has an innovative and diversified portfolio of next-generation technologies.
- The company has seven novel assets in the fast-growing segment of oncology drug development.
- CHM says that three assets are undergoing clinical trials, with initial positive signals from two phase 1 clinical trials.
- Chimeric expects to hit multiple key clinical milestones over the next 12-24 months.
Chimeric Therapeutics (ASX:CHM) is moving forward with full gusto to bring the promise of cell therapy to life. The ASX-listed clinical-stage company believes that novel cell therapies hold the potential to cure cancer.
Chimeric has one of Australia’s most robust and advanced cell therapy portfolios and pipelines. It has seven assets under development focusing on 10 disease areas. The company has three ongoing clinical trials, with two trials recording early positive clinical signals.
© 2022 Kalkine Media®, data source: company update
Cell therapy – A multi-billion-dollar market opportunity
Cell therapy is expected to be the fastest-growing market in the oncology space. Chimeric referring to industry reports suggest that the market size of cell therapy is projected to grow from US$10.69 billion in 2022 (oncology market:US$265 billion) to US$42.37 billion in 2027 (oncology market:US$581 billion), representing a compound annual growth rate (CAGR) of 31.7%.

© 2022 Kalkine Media®, data source: company update
Chimeric’s Cell Therapy portfolio
Chimeric has a broad portfolio with cutting-edge innovation and huge commercial opportunities. The portfolio includes NK cell-derived allogenic therapies and T cell-derived autologous therapies.

Image source: company update
Natural killer (NK) cell therapy - Natural Killer (NK) cells are part of our innate immune system providing mechanisms that can kill cancer. They are ideal foundations for the development of off-the-shelf (allogeneic) cell therapies. These cells also have fail-safe mechanisms that inhibit the killing of healthy cells, making them ideal for off-the-shelf platforms.
NK cell therapy has a comparatively healthier starting material with a scalable production process at reduced costs. It is rapidly available, off the shelf and can reach more patients.
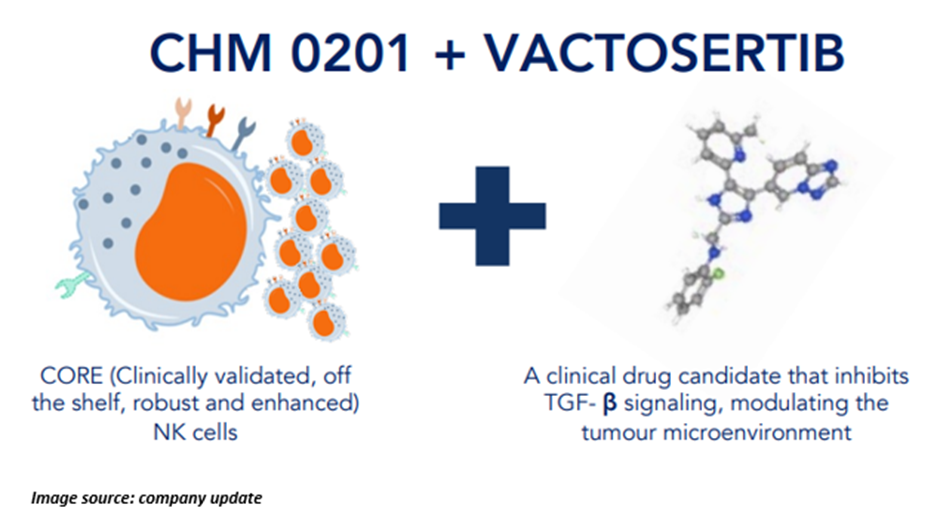
As per CHM, CHM 0201, a clinically validated, off-the-shelf NK cell platform, demonstrated promising results with safety, persistence, and expansion in Phase 1 study across all key points. It showed a 33% disease control rate in solid tumours and a 100% disease control rate in blood cancers, says the company.
In June 2022, CHM announced conducting the first-ever trial of NK cells. The trial was focused on the study of these cells in combination with Vactosertib and IL-2. With approval secured from the US FDA, the Phase IB investigator-initiated trial will recruit 12 patients with either relapsed/refractory blood cancers or locally advanced/metastatic colorectal cancer. The study is likely to be concluded by December next year.
The study aims to identify a combination therapy that could enhance the initial efficacy signal seen with CHM 0201 (CORE NK) cells by modulating the tumour microenvironment.
T cell-derived autologous therapies
T cells are part of our adaptive immune system that modifies the body’s response to specific pathogens. They do not generically attack any antigens but circulate until they encounter a particular antigen. They play an important part in immunity against foreign substances. T cell-derived therapies have proven efficacy in blood cancers.
Novel and promising CAR T therapy CHM 1101 (CLTX CAR T) is being studied against solid tumours. It demonstrated encouraging initial data in glioblastoma with a 70% disease control rate and safety with dual routes of administration.
Chimeric has planned a 2nd CLTX CAR T phase 1 clinical trial in metastatic melanoma with studies to be expanded in additional solid tumours in the future.
In August, the company announced securing a Japanese patent for CHM 1101.
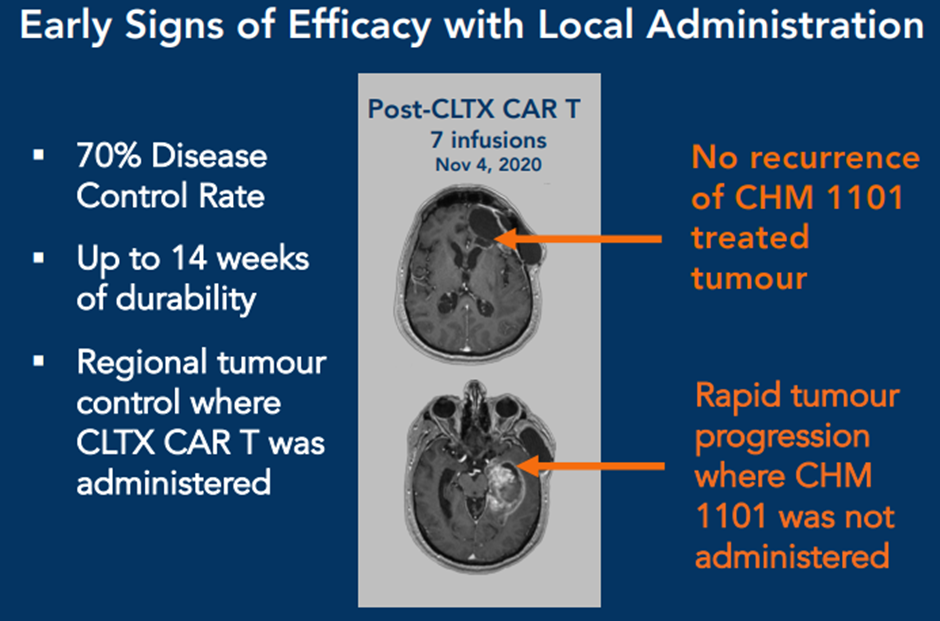
Image source: company update
CHM 2101 - CDH17 CAR T Cell Therapy was invented by the University of Pennsylvania, a cell therapy centre well-known across the globe. Preclinical evidence for the novel, 3rd generation asset was published in the first quarter of this year in a highly prestigious journal, Nature Cancer. It showed complete suppression of tumour cells with no relapse and high tumour-specific activity in vivo with no on-target / off-tumour toxicity.
The past 12 months have been a year of major milestones for the company. Let us have a look:
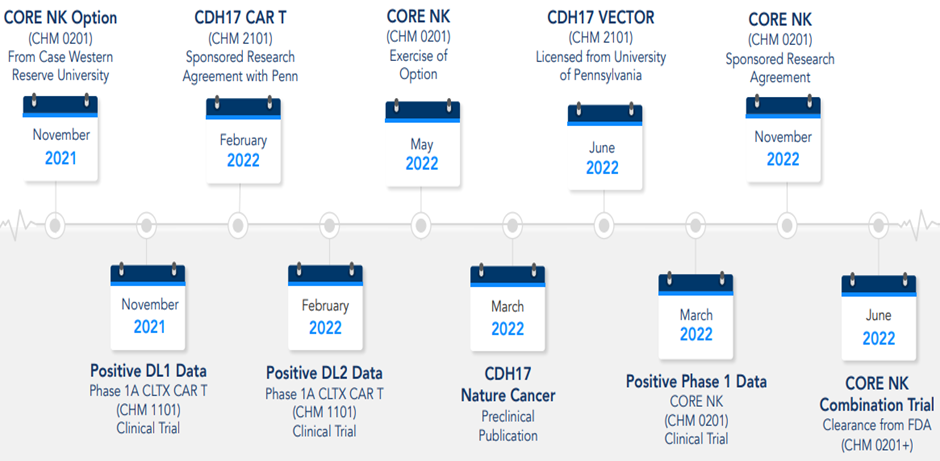
Image source: company update
The way forward
In the next 6-12 months, Chimeric expects to achieve many key milestones including asset production, initiation of several trials, securing FDA clearances, and implementation of agreements and partnerships.
CHM shares traded at AU$0.079 on 20 December 2022.