Highlights
- Chimeric presented the CHM 1101 Phase 1B clinical trial design and objections at the American Society of Clinical Oncology meeting in Chicago
- Abstract gave background data on one patient from initial dose cohort (survival of 15.5 months from time of first infusion)
- Chimeric's multi-site CHM 1101 Phase 1B trial is being done under a US IND and progresses on the Phase 1A dose escalation study
Chimeric Therapeutics (ASX: CHM) -- a listed cell therapy entity -- has informed that the company last week presented the design and objectives of its new CHM 1101 Phase 1B clinical trial (glioblastoma multiforme) at the American Society of Clinical Oncology (ASCO) 2023 meeting held in Chicago, US. Notably, this latest phase of clinical trial by CHM progresses on the Phase 1A dose escalation trial (no dose limiting toxicities) and a formerly presented 75% disease control rate.
The Phase 1B -- under a US IND -- is a multi-site trial of CHM 1101 (a first-in-class CAR T therapy), and Chimeric has stated that it is planning to assess the safety and activity from the cell therapy's clinical program later this year.
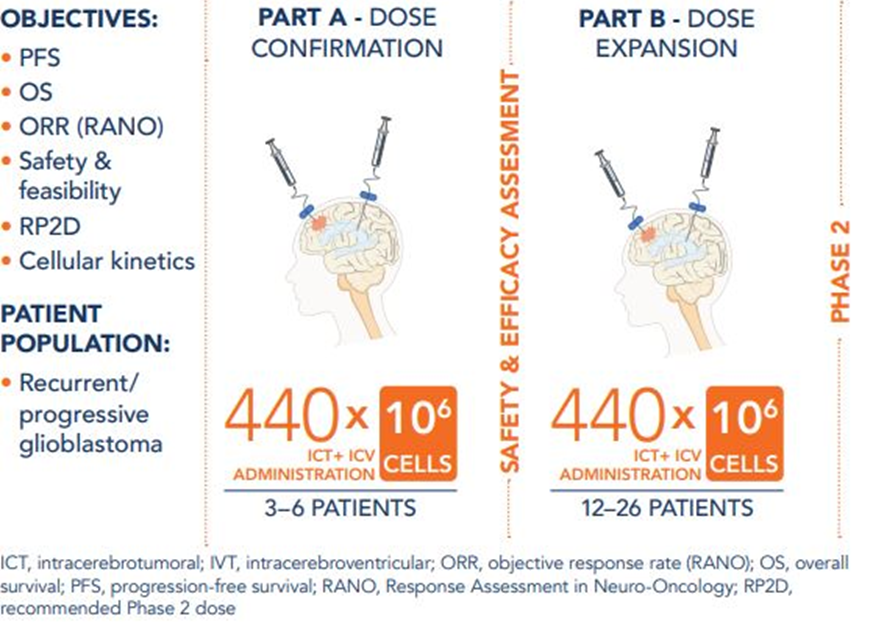
Source: Company update
More
Chimeric Therapeutics -- listed on Australian stock exchange ASX under the ticker CHM -- last week (3 June 2023) presented its cell therapy CHM 1101 Phase 1B clinical trial at the ASCO Annual Scientific Meeting 2023. The meeting was scheduled between 2 June and 6 June in Chicago, US. Chimeric provided the design and objectives of the new Phase 1B trial -- now open for enrolment at St. David’s South Austin Medical Center (Austin, Texas) -- at the event.
It is pertinent to note that the Phase 1B of CHM 1101 (under a US IND) is with respect to people suffering from recurrent and/ or progressive glioblastoma multiforme. The trial is designed to have two parts. The first part involves enrolment of three to six patients who will be administered CHM 1101, while the second (Part B) will be a dose expansion cohort with enrolment of further 12 to 26 patients.
Chimeric has mentioned that the abstract presented at the event was about background data on one patient treated in the initial dose cohort. The patient survived 15.5 months from the time of first infusion. This has been termed "compelling" by CHM that is because patients with recurrent/ progressive glioblastoma are "generally expected” to have survival for two to nine months.
About Chimeric and CHM 1101
Chimeric Therapeutics is an Australian cell therapy company with a diversified portfolio of CAR T and NK cell therapies. CHM 1101 is a CLTX CAR T therapy that is being developed for the treatment of solid tumours. The investigator-initiated phase 1A trial provided initial positive data. It was related to patients who were treated with first two dose levels.