As the cannabis sector gains momentum in Australia and given that medicinal cannabis was legalised in February 2016, at a federal level, various companies are rapidly advancing their clinical trials for relevant products to capitalise on this boom and contribute to the treatment of small as well as severe diseases and medical conditions. Letâs take a look at how Zelda Therapeutics and Botanix Pharmaceuticals, both with stupendous positive return yields are progressing with their endeavours in this line.
Zelda Therapeutics Limited
The Australia-based bio-pharmaceutical company, Zelda Therapeutics Limited (ASX: ZLD) is engaged in the formulation and development of a range of cannabinoid-based products to treat different medical conditions. Currently, Zelda is carrying out a human clinical trial programme for the treatment of autism, insomnia and reducing opioid with activities across Australia and the USA; and also, a pre-clinical research programme assessing the effectiveness of cannabinoids in treating brain, breast and pancreatic cancer. Besides, it is also conducting research to examine the potential for cannabinoids in the treatment of diabetes-related cognitive decline.
 Source: Investor Presentation
Source: Investor Presentation
With a market capitalisation of around AUD 49.85 million with approximately 755.34 million shares outstanding, the ZLD stock is trading today, (11 July 2019, AEST 12: 19 PM) at AUD 0.063, down 4.54% by AUD 0.003, with approximately 2.31 million shares traded. In addition, the ZLD has generated a positive return of 57.14% in the past one-month.
Today, Zelda Therapeutics has also reverted to a query lodged by the ASX on 10 July 2019, stating that the company has not announced any information that could explain the recent change in the price of its securities from a low of $ 0.046 at close on 8 July 2019 to a high of $ 0.067 midday on 10 July 2019 or the significant increase in the volume of securities traded. In addition, the company has mentioned that the prior announcement on 2 July 2019, regarding the formal approval for the Phase I Opioid Reduction Trial by the St Vincentâs Hospital Ethics and Governance Committees could have been a catalyst in strengthening the investorâs confidence.
Phase I trial has already commenced, whereby the safety and tolerability of whole plant extract following single and repeated doses in nine patients with chronic non-cancer pain on long-term opioid analgesia will be evaluated. The preliminary results for the same are expected to be released by late Q3 2019 and final results in Q4 2019, which is similar to the timeframe the Company expects to report on its ongoing Phase II Insomnia trial.
Moreover, Zelda Therapeuticsâ CEO has also made several presentations explaining the Companyâs business and its objectives, which have been well attended by investors.
Directorâs shareholding - On 11 June 2019, the company updated the market that the Director Ms Mara Gordon has sold a total of 35,500,000 shares to settle personal commitments and helped facilitate a major investment in Zelda by a strategic investor. A total of 26,931,660 of these shares have been acquired by leading boutique investment management group Merchant Funds Management, which was an early mover into the Australian medical cannabis sector and has had a long association with some of the Founders of Zelda.
Following this transaction, Ms Gordonâs now holds 44,142,326 shares while she remains on the Board of the Company (Non-executive Director). The Board of Directors own 27% of issued capital in the company.
R&D Tax Incentive Refund- Zelda Therapeutics also received an R&D $ 769k cash refund under the Governmentâs R&D Tax Incentive Scheme in early May 2019.
Botanix Pharmaceuticals Ltd
Botanix Pharmaceuticals Ltd (ASX:BOT), is a clinical stage cannabis sector company based in Perth, Australia) and also in Philadelphia, USA, and diligently working to develop pharmaceutical products (see figure below) for the treatment of serious skin diseases including psoriasis, acne and atopic dermatitis.
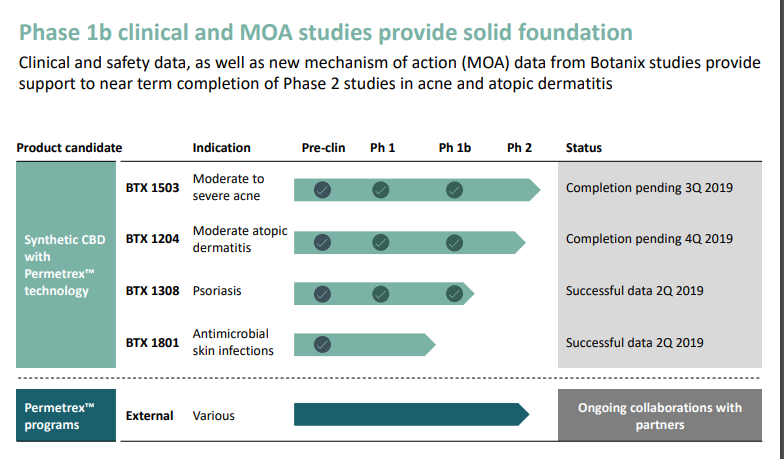 Source: Updated investor presentation 20 June 2019
Source: Updated investor presentation 20 June 2019
Botanix also has an exclusive license to use a proprietary drug delivery system (PermetrexTM) for direct skin delivery of active pharmaceuticals in all skin diseases and is working with multiple parties to test the application of it on both a fee-for-service and traditional license basis.
Botanix Pharmaceuticals conducts business worldwide and has a market cap of ~AUD 201.25 million with approximately 774.03 million outstanding shares. On 11 July 2019 at AEST 01:29, the BOT stock was trading at AUD 0.275, up 5.77% with around 6.63 million shares traded till that time.
In addition, the stock has delivered an impressive return of 147.62% for the past one-month.
Recent updates - Recently on 27 June 2019, the company announced that Vince Ippolito, Botanix President, was recently interviewed by the Finance News Network. In the interview, Mr Ippolito provided details on his extensive experience in medical dermatology and also highlighted the clinical and commercial importance of Botanixâs BTX 1308 mechanism of action data and BTX 1801 antimicrobial data.
Prior to that on 24 June 2019, Botanix Pharmaceuticals informed that Dr Mark Blaskovich of the University of Queensland (collaborator of BOT) presented new antimicrobial data at ASM Microbe 2019, highlighting cannabidiol as a remarkably active Gram-Positive antibiotic. Dr Blaskovichâs poster presentation was particularly highlighted as the significant new research and the conference team separately conducted a press release. Thus, antimicrobial resistance provides a major opportunity and the BTX 1801 data validates Botanixâs position as a leading developer of treatments for skin disease.
Investor presentation with new BTX 1801 data- On 20 June 2019, Botanix Pharmaceuticals disclosed to the market an updated investor presentation elaborating on the key observations and inferences of the BTX 1801 antimicrobial study, which showed potential for BTX 1801 to be significant in treating serious skin infections. The key findings includeâ
- Cannabidiol containing BTX 1801 rapidly kills Gram-Positive Staphylococcus aureus (staph) and methicillin-resistant Staphylococcus aureus (MRSA).
- The data showed that MRSA superbugs did not develop resistance even when they were extensively exposed to cannabidiol.
- In addition, the data also supported the anti-inflammatory, immune cell modulating and antimicrobial nature of cannabidiol, highlighting the potential of Botanix development products to successfully address a range of skin diseases.
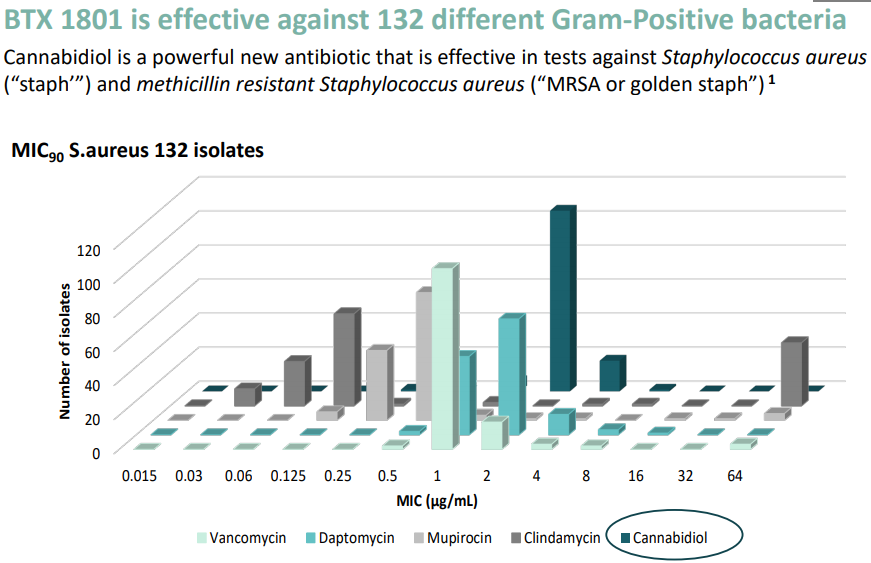 Source: Updated investor presentation 20 June 2019
Source: Updated investor presentation 20 June 2019
Psoriasis market and BTX 1308- The company, on 14 June 2019, released a review of market opportunities in psoriasis for BTX 1308, analysing the limitation of the current products and the potential to fill the gap through the introduction of its novel topical agent. The Advisory Meeting was held with the Companyâs key opinion leaders in Milan, Italy, in conjunction with Botanixâs attendance at the 24th World Congress of Dermatology.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. Kalkine Media does not in any way endorse or recommend individuals, products or services that may be discussed on this site. Our publications are NOT a solicitation or recommendation to buy, sell or hold. We are neither licensed nor qualified to provide investment advice.




_09_03_2024_01_03_36_873870.jpg)
