Highlights
- In healthcare, real-word data (RWD) is the data relating to a patient’s health status and delivery of health care.
- Biotech and pharmaceutical companies are using RWD and real-world evidence (RWE) for supporting clinical trial designs.
- RWD data help companies in designing clinical trials, study site selection and remote patient monitoring, among others.
Real-world data (RWD) in healthcare is the data related to the patient health status as well as the delivery of health care. It is routinely gathered from various sources, for example, electronic health records (EHRs), claims and billing services, patient generated data.
Medical product developers are using RWD and real-world evidence (RWE) for supporting clinical trial designs (such as pragmatic clinical trials, large simple trials) as well as observational studies for generating innovative and new treatment methodologies.
With real-world data, investigators will be able to go beyond the scope of conventional clinical trials, obtaining insights from the information collected in routine clinical care.
RELATED ARTICLE: Electronic Medical Records (EMR): What are they, and why do we need them?
There are many different types and sources of real-world data; below are few types and sources of real-world data-
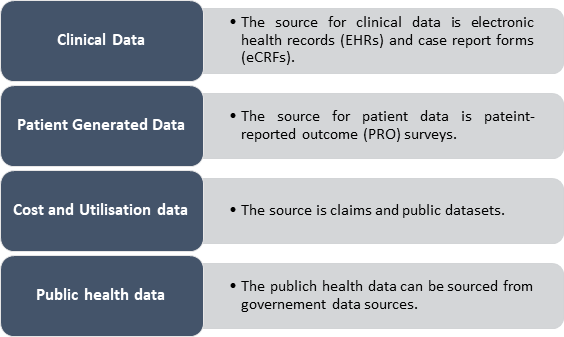
Copyright © 2021 Kalkine Media (Data Source: Arbormetrix)
RELATED ARTICLE: What is predictive analytics and how is it shaping the healthcare industry?
Need of real-world data in clinical trials
Healthcare sector companies are dealing with unprecedented cost pressures, and the cost associated with drug development is steadily increasing. The use of RWD can encourage healthcare companies to respond to these challenges.
RWD consists of real patient data from EHRs, patient registries, surveys, and data from smartphones and wearable devices. It can help investigators to accurately assess the safety as well as the efficacy of new treatments during clinical studies. Thereby enhancing the prospects of success of the drug and return on investment in R&D.
By enabling the data analysis of the real-world use of medicines, real-world data can generate real-world evidence (RWE). Notably, access to significant RWE can accelerate the process of getting approvals from health plans as well as lower the time for the launch of a new drug.
Furthermore, with RWE, researchers can examine which medications are suitable for certain patients, leading to personalised care and improved patient outcomes.
Let us now discuss the use of real-world data in the process of drug development-
Designing more efficient and successful clinical trials
For designing of any clinical trials, the drug development groups should set criteria for inclusion and exclusion of different patients. Real-world data can exhibit the impact of different criteria on the potential group of patients. Moreover, using RWD optimisation criteria can help accelerate the process of patient recruitment and ensure that the trial findings are more relevant.
Clinical trial site selection
Once the protocol is designed for any clinical trial, the study should be performed only in those sites where there is evidence of availability of eligible patients. Real-world data system has a built-in communication feature to the clinical trial sites and helps the user to select sites with pre-screened patients.
Remote monitoring of patients
Traditional clinical trials have relied upon electronic patient-reported outcomes (ePRO) for capturing data. ePRO can offer valuable insights on patient perceptions as well as the quality of life. Furthermore, with the use of wearables and devices, healthcare service providers and clinical trial investigators can now monitor clinical-grade vital signs remotely.
RELATED ARTICLE: Virtual care is booming amid pandemic: A glance at the pros and cons of telehealth
Protecting the safety of patients
Real-world data is commonly used to augment clinical trial data for the determination of the safety profile of medicines. The data can be particularly useful in the determination of the risk of rare but potentially serious side effects.
What’s ahead?
Healthcare companies engaged in clinical trials should focus on identifying new prospects to innovate and enhance operational competencies, patient experience as well as clinical outcomes. In addition, the companies should leverage RWD and RWE to drive new methodologies for providing high-value insights into their R&D and commercial efforts.



