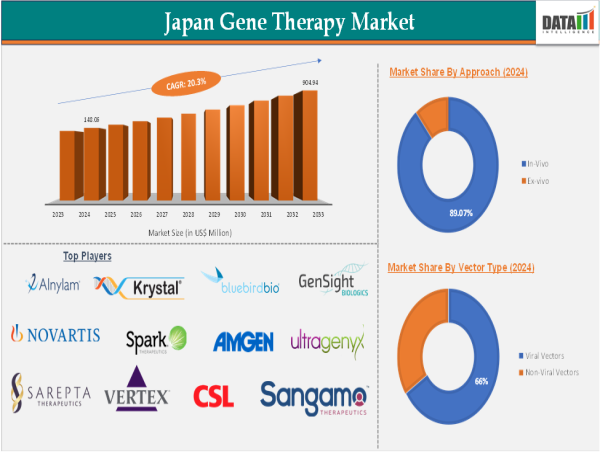
In 2024, the Japan Gene Therapy Market Size was valued at US$ 148.06 Million in 2024 and is forecasted to see substantial growth, reaching around US$ 904.94 Million by 2033, with an anticipated CAGR of 20.3% during the period from 2025 to 2033.
To Download Sample Report: https://www.datamintelligence.com/download-sample/japan-gene-therapy-market
Regional Outlook
Gene therapy is gaining ground across Japan’s major regions:
Kanto (Tokyo area) is home to the country’s top biotech startups and major pharmaceutical headquarters, where most clinical trials and regulatory activities are coordinated.
Kansai (Osaka, Kyoto) is becoming a center for regenerative medicine and gene editing research.
Kyushu and Tohoku are witnessing the emergence of research hubs focused on neurological and inherited diseases.
Chubu and Hokkaido are showing interest in gene therapy logistics and delivery systems, including ultra-cold storage and transportation networks.
This regional expansion minimizes bottlenecks and encourages localized innovation, positioning Japan as a leader in the APAC gene therapy market.
Company Landscape
Alnylam Pharmaceuticals, Inc.
NOVARTIS AG
Sarepta Therapeutics, Inc.
Krystal Biotech, Inc.
CSL
Bluebird Bio, Inc.
SPARK THERAPEUTICS, INC.
Ferring
Vertex Pharmaceuticals Incorporated
Amgen, Inc
Orchard Therapeutics Plc
Gensight Biologics
Ultragenyx Pharmaceutical Inc.
REGENXBIO Inc.
Oxford Biomedica PLC
Benitec Biopharma Inc.
Transgene
Sangamo Therapeutics
Market Segmentation:
By Approach: In-Vivo, Ex vivo
By Vector Type: Viral Vectors, Adeno-Associated Virus , Herpes Simplex Virus, Lentivirus, Non-Viral Vectors
By Technique: Gene Addition, Gene Silencing, Gene Editing
By Application: Rare Diseases, Musculoskeletal Conditions, Blood Disorders, Oncology, Ophthalmology, Others
Buy Now: https://www.datamintelligence.com/buy-now-page?report=japan-gene-therapy-market
Latest Developments
In July 2023, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) approved Novartis' Luxturna to treat inherited retinal dystrophies (IRDs) caused by biallelic mutations in the RPE65 gene. This gene therapy is now accessible in Japan for individuals affected by this rare genetic disorder.
Latest News from Japan
New Approval for Rare Disease Treatment
Japan’s drug regulators recently approved a new gene therapy for children suffering from a rare form of muscular dystrophy. This treatment is designed for a specific subset of patients and has been approved with a limited license for ongoing data collection. Japan’s conditional approval process allows patients faster access to innovative therapies while ensuring long-term safety and effectiveness.
Expansion of Clinical Trials
More gene therapy clinical trials are being launched in Japan than ever before. Hospitals and research institutions are now working with pharmaceutical partners to test new therapies for inherited blood disorders, eye diseases, and neurological conditions. This increase in trials is helping to build a stronger evidence base and more local data for regulatory decisions.
Cold Chain Innovations
Japanese companies are also leading the way in solving one of gene therapy’s toughest logistical challenges: maintaining ultra-cold storage conditions during transit. A successful long-distance delivery of sensitive gene therapy products, maintained at sub-zero temperatures, was recently completed, demonstrating Japan’s readiness for global therapy distribution.
Experts Thoughts:
The gene therapy market in Japan is no longer a future vision it’s a thriving, rapidly growing field that holds promise for transforming how chronic and rare diseases are treated. With strong government support, world-class researchers, and an eager medical community, Japan is uniquely positioned to become a global leader in gene therapy development.
The parallel developments in the U.S. reinforce the idea that gene therapy is going global, and Japan is playing a crucial role in this transformation. From regulatory speed to scientific rigor and infrastructure readiness, Japan is laying the foundation for a healthier, gene-powered future.
Here Are The Latest Related Report Experts Researched By DataM intelligence
Gene Therapy Market Size 2025-2033
Cancer Gene Therapy Market Size
Buy Now & Unlock 360° Market Intelligence:
Purchase Industry Subscription Today – Make Smarter Decisions Tomorrow:
Purchase Your Subscription to Power Your Strategy with Precision: https://www.datamintelligence.com/reports-subscription
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
[email protected]
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()