SUDA Pharmaceuticals Ltd (ASX:SUD), an oro-mucosal drug delivery company headquartered in Perth, focusses on developing low-risk oral sprays using its proprietary OroMist® oro-mucosal technology to reformulate the existing medications. The OroMist® technology enables the delivery of drugs via gums, cheeks, the floor of the mouth or tongue, thus minimising the dose variation related to stomach emptying time, enzymatic degradation in the gut, tablet/capsule disintegration and dissolution, food effects and gastrointestinal tract motility.
In a recent update on the Australian Stock Exchange, SUDA notified that Dr Richard Franklin has joined the company as Project Director for the development of anti-thrombotic agent, Anagrelide. The company informed that Dr Franklin will fully direct the Anagrelide project till the completion of Phase I clinical studies. He will be work with the companyâs project team and involve with key opinion leaders and world experts on Anagrelide. He was working with SUDAâs project team and management last week in Perth.

Dr Richard Franklinâs Experience
Currently a member of the companyâs Science Advisory Board, UK-based Dr Franklin holds more than forty years of experience in pharmaceutical research & development. Dr Franklin has previously worked as a Research Fellow & New Product Innovation Head (small molecules) at US-headquartered Shire Pharmaceuticals, where he is credited with lodging more than 40 patents on potential new drug products. He has also worked with Sterling Winthrop, Wyeth, AstraZeneca and Glaxo in senior roles. Besides this, he was also the Associate Editor of the journal Xenobiotica for ten years and was the secretary and chairman of the European Drug Metabolism Discussion Group. He has published more than 60 scientific papers during his career.
Dr Franklinâs drug development experience covers a broad range of therapeutic areas and different products. Dr Franklin was earlier involved in the EU registration and development of Anagrelide as Xagrid® for the treatment of Essential Thrombocythemia, the orphan drug condition.
SUDA believes that Dr Franklin is well qualified to lead this thrilling new venture in utilising Anagrelide in the fight against cancer.
SUDAâs Anagrelide Project
Anagrelide is an approved generic drug for treating a rare blood disorder where a patientâs platelet count is too high. The companyâs Anagrelide project is related to the use of a completely new and unique approach to treat cancer, potentially pertinent over a broad range of solid tumours. As per SUDA, Anagrelide could be used in conjunction with treatments that involve immunotherapy stimulating the patientsâ own immune system, rendering circulating cancer cells more vulnerable to attack by the body's own "killer" T cells. Like the immunotherapy process, Anagrelide research has also demonstrated that it âawakens,â the immune system to enjoin the fight against the attacking cancer cells.
The research conducted so far has shown that Anagrelide lowers the elevated platelets and thrombotic threat in myeloproliferative diseases resulting from large platelet counts. It is a beneficial outcome as platelets are recognised to play a significant role in the metastatic spread and growth of cancerous tumours. There is substantial evidence available in the scientific community, which verifies that reducing the platelet count can help improve outcomes for cancer patients.
As per the company, Anagrelide has some cardiac side effects, but if delivered via SUDAâs oro-mucosal spray, this toxicity is potentially avoided. The reformulation work for an OroMist spray of Anagrelide is currently underway at SUDA. The company owns the intellectual property for the use of Anagrelide in cancer titled, âPrevention and treatment of metastatic disease in thrombocytotic cancer patientsâ.
Recent Updates on SUDAâs Projects
SUDAâs ArTiMist® Appeal Filed with TGA
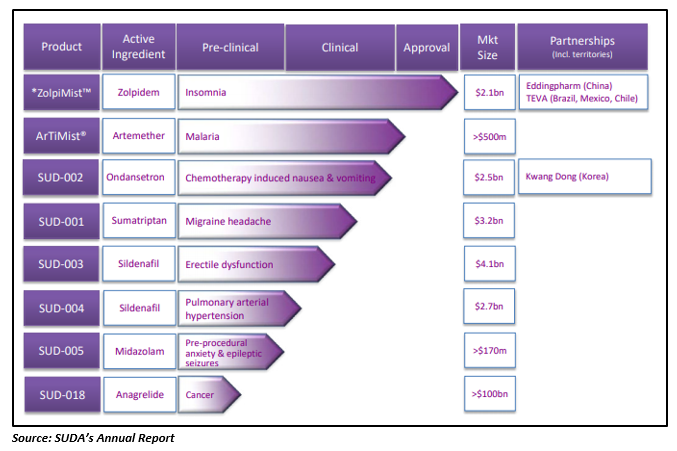
Recently, SUDA also released an update on its another product under development, ArTiMist® oral spray for the treatment of malaria. SUDA notified that the Department of Healthâs Division, the Australian Therapeutic Goods Administration (TGA) has provided the acknowledgement of receipt of the application regarding reconsideration of the marketing approval of its ArTiMist® oral spray, which was earlier denied by Advisory Committee of Medicines in April 2019. In accordance with section 60(4) of the Therapeutic Goods Act 1989, the Ministerâs delegate must notify the company in writing over the decision on reconsideration, within 60 days from the date of receipt by the Minister i.e. on 4th October 2019.
ZolpiMistTM Clinical Results Out
SUDA recently announced the clinical study results of its USFDA approved insomnia drug, ZolpiMistTM oral spray early in August. The clinical study results published in the journal Pharmacy and Pharmacology demonstrated that ZolpiMistTM lingual spray achieves a more rapid onset of sleep relative to Zolpidemâs tablet form. In comparison to Zolpidemâs tablet form, the lingual spray ZolpiMistTM achieved blood serum therapeutic threshold faster, succeeded in DSST Test and resulted in increased bioavailability.
EPO Intended to Grant Anagrelide Patent
Last month, SUDA also received an information from the European Patent Office (EPO) about its intention to grant SUDAâs Anagrelide Patent. The company mentioned that EPO would grant the patent âUse of Anagrelide for Treating Cancerâ with Application No. 15817516.6 and Expiry Date - December 2035. The granting of the patent was considered to be a noteworthy step in the SUDAâs commercialisation process and confirmed its position as the only company to have a patent covering the use of Anagrelide for the prevention or treatment of metastatic disease in the lung or bone in a patient with solid cancers and a high platelet count.
SUDA is using its OroMist® oro-mucosal technology for the redevelopment of the existing billion-dollar drugs for oral delivery, ensuring faster onset, potentially smaller doses & safer delivery for ex-blockbuster drugs. The reformulation presents a promising opportunity for the company to take a drug approved for blood disorder & convert it to an oro-mucosal spray cancer drug.
Stock Performance
SUD is currently trading at AUD 0.005, up 25% by the close of trading session on 13th August 2019. The stock has delivered enormous returns of 50 per cent and 18.42 per cent in the last one month and three months, respectively.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. The above article is sponsored but NOT a solicitation or recommendation to buy, sell or hold the stock of the company (or companies) under discussion. We are neither licensed nor qualified to provide investment advice through this platform.