SUDA Pharmaceuticals Limited (ASX:SUD), an Australia-based oro-mucosal drug delivery player, notified in its latest update that the Therapeutic Goods Administration (TGA) review for its insomnia drug ZolpiMistTM is expected to be completed by Q4 2020, subject to the TGA feedback.
TGA is reviewing additional data submitted by SUDA for ZolpiMistTM, which supports the quality of the product being produced using the new active pharmaceutical ingredient (API) supplier and manufacturer.
Post the key update, SUDA’s stock surged by ~6.5 per cent on the ASX to $0.049 on 12th May 2020.
A first-in-class oral spray of zolpidem for insomnia, ZolpiMistTM is the most advanced product of SUDA, which has demonstrated faster sleep onset than zolpidem tablet form in the clinical study. ZolpiMistTM oral spray was approved by the FDA in 2008, while the TGA submission for approval is ongoing in Australia.
Must Read! SUDA’s March Quarter Report Card Out! Let’s Browse Through Key Developments
Additional Data Submitted to TGA, Review in Progress
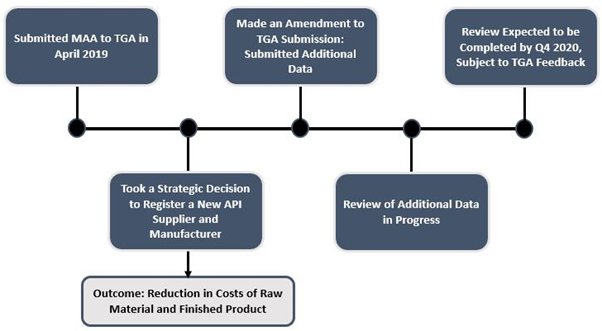
SUDA took a strategic decision to register a new API supplier and manufacturer, after the submission of Marketing Authorisation Application (MAA) for ZolpiMistTM to the TGA in April 2019.
The result of SUDA’s strategic decision is a decline in costs of raw material and thereby finished product, which will allow the Company to generate additional value from its existing partnerships over the medium to long-term and establish additional partnerships across further territories.
SUDA will now source the API from a renowned global generics manufacturer, while the production and packaging of ZolpiMistTM will be done by an Australian subsidiary of a popular international manufacturer of generic prescription and OTC (over-the-counter) medications.
The strategic decision of registering a new API supplier and manufacturer demanded an amendment to the TGA submission. To support this amendment, the Company submitted additional data to the TGA endorsing the quality of the product being produced using the new API supplier and manufacturer, that is being reviewed.
SUDA intends to share this additional data with its existing and potential partners to back their regulatory submissions.
SUDA Continues to Make Great Strides with ZolpiMistTM
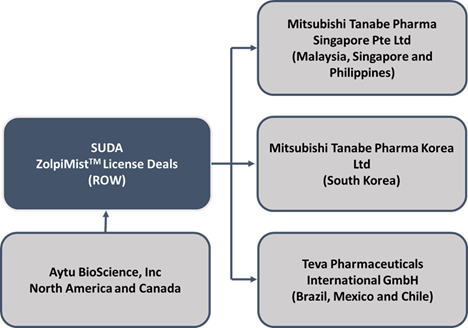
SUDA continues to make progress with the commercial buildout of ZolpiMistTM, securing additional license and supply agreements for the product. For ZolpiMistTM, SUDA has recently signed an additional license agreement with one of the top pharmaceutical companies of Japan, Mitsubishi Tanabe Pharma Korea Ltd (MTPK).
In addition to MTPK, SUDA has signed license and supply deals with several other pharma players for ZolpiMistTM, as demonstrated in the figure below:
In line with its strategy for global commercialisation of ZolpiMistTM, SUDA is continuing discussions with various pharma companies covering a range of territories,
Notably, SUDA holds considerable potential to expand commercial buildout of ZolpiMistTM, for which it has rights outside of Canada and the US. In addition to the insomnia market, the Company is ideally positioned to leverage its solid network across other large target markets including cancer, migraine and medical grade cannabis.