Highlights
- Radiopharm’s progress during the June quarter was marked by major agreements, technology additions and new appointments.
- The company gained access to brain tumour technology, high-quality medical isotope, and promising LRRC15 antibody.
- RAD roped in three key officials and remained well funded to accelerate its clinical programs.
Radiopharm Theranostics (ASX:RAD) saw a super busy June 2022 quarter with major developments across its clinical portfolio and new appointments to its world-class team.
A major highlight subsequent to the period end was the company’s extended agreement with GenesisCare to accelerate a prostate cancer clinical trial in Australia.
At the quarter-end, the company had cash and equivalents worth AU$27.0 million.
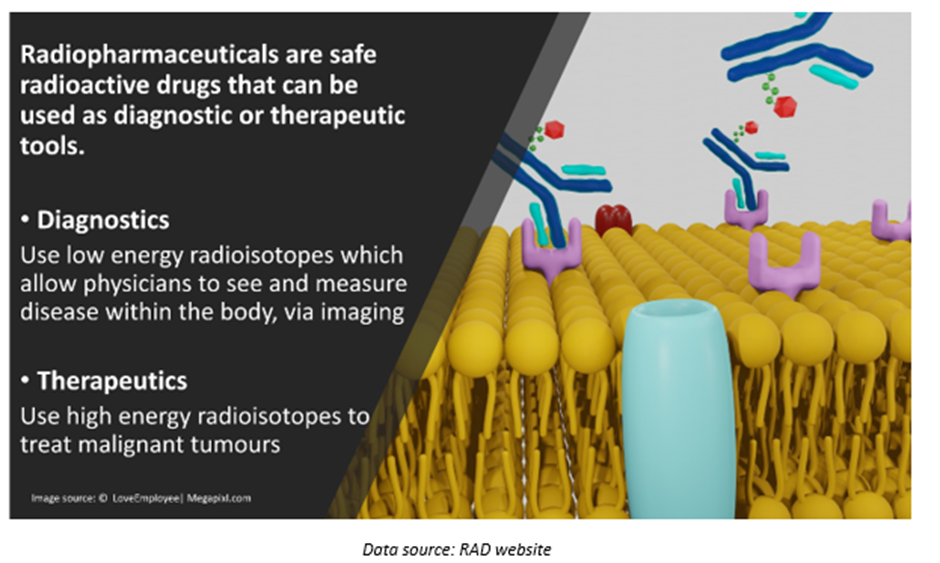
The ASX-listed oncology space player is committed to developing a world-class platform of radiopharmaceutical products.
Strengthened portfolio with the addition of brain tumour technology
Radiopharm signed an exclusive sublicensing agreement with NeoIndicate, LLC, gaining rights to develop a PTPμ-targeted radiopharmaceutical agent.
Depending upon the level of radiation, PTPμ-targeting agent can function both as an imaging agent and as a radiopharmaceutical theranostic to destroy tumours.
The technology has exhibited positive pre-clinical data in human glioblastoma (the most common and destructive form of brain cancer).
The company intends to start manufacturing PTPμ in late-2022.
During mid-June, Radiopharm sealed a pact with Isotopia Molecular Imaging, under which the latter will supply high-quality Lutetium-177 N.C.A.
Lutetium-177 N.C.A is a crucial isotope necessary for several clinical trials in Radiopharm's clinical pipeline. This agreement will assist Radiopharm in advancing its next generation of radiopharmaceutical therapies for cancer treatment.
Radiopharm plans to use Lutetium-177 N.C.A in clinical research, development, production, and early-stage commercialisation of its diagnostic and therapeutic products.
Licenced dual-action antibody from UCLA
The quarter saw an exclusive licensing agreement with the University of California Los Angeles (UCLA) Technology Development Group (UCLA-TDG).
Under the deal, Radiopharm licenced "DUNP19", a first-of-its-kind therapy and UCLA's promising LRRC15 antibody. The company now holds the rights to use this antibody as an Antibody-Drug Conjugates (ADC) within radiotherapy as part of its clinical development pipeline.
Extended deal for Australian Prostate Cancer Trial
Subsequent to the reported period, Radiopharm expanded its agreement with GenesisCare, a global oncology player.
The extended agreement will support a therapeutic phase 1 clinical trial related to prostate cancer, expected to start in the coming months. Under the study, the company plans to use its prostate kallikrein (PSA) targeting antibody, which is a novel and pioneering mode of action compared to other currently under development treatments.
Crucial team additions to accelerate clinical studies
During the quarter, Radiopharm strengthened its team with three new additions.

Data source: company reports
Radiopharm has a market capitalisation of AU$53.64 million, and its shares traded at AU$0.21 on 8 August 2022.





