In the world of cancer, one billion-dollar molecule that has been making a big noise for its potential to extend life in men with the late-stage prostate cancer, concerns an experimental radioactive drug called Lutetium-177 (177Lu) labelled PSMA-617 (referred to as 177Lu-PSMA-617). This molecule is being investigated for its ability to give some extra time with less pain to the men with prostate cancer, where the cancer is progressive and at the last stage with mounting pain from multiple secondary tumours growing predominantly in the skeleton.
The biotech industry might have made many developments in the recent past, but the treatment for a common cancer like late-stage prostate cancer still remains largely unmet. For a company like Noxopharm Limited (ASX: NOX), their research and development may mean a huge opportunity in the global pharmaceutical industry.
In one of its trials, Noxopharm has placed its belief in the synergies of radiopharmaceutical 177Lu-PSMA-617 and its lead drug candidate Veyonda® to get started with the investigator initiated clinical trial called LuPIN in Australia. This ongoing trial, initiated by St Vincentâs Hospital in Sydney, focuses on a therapeutic approach where the radioactive drug Lu-PSMA supplied by US biotech company, Endocyte is injected intravenously to spread across the prostate cancer cells everywhere in the body. And the combination of Veyonda® with this radioactive drug is being investigated to see if Veyonda® will boost the effectiveness of the radiation, so that more men respond and stay on treatment longer under the well-tolerated environment.
Good News for Investors: LuPIN Trial showing encouraging interim data
St Vincentâs unveiled the results that outlined the highly encouraging interim data from its ongoing LuPIN study with Noxopharm, in a presentation at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in their 2019 Annual Meeting held in California from 22 to 25 June.
The Principal Investigator of the study and Director of Theranostics and Nuclear Medicine at St Vincentâs Hospital, Associate Professor Louise Emmett presented the interim data at the conference for the first 16 patients evaluated under the 56-patients LuPIN Study from the Phase Ib/IIa trial. The data reported an 69% overall response rate, (exceeding the target of >50% reduction in PSA); with over half of the men in this first group (9/16) being able to complete their full course of 6-cycles of treatment, all in a generally well-tolerated way.
About this data: This research was originally published in an earlier form in the Journal of Nuclear Medicine. Emmett L et al. Interim results of a Phase I/II prospective dose escalation trial evaluation safety and efficacy of combination 177Lu-PSMA-617 and NOX66 in men with mCRPC post androgen signaling inhibition and 2 lines of taxane chemotherapy (LuPIN trial). The publication is available at the following link: #
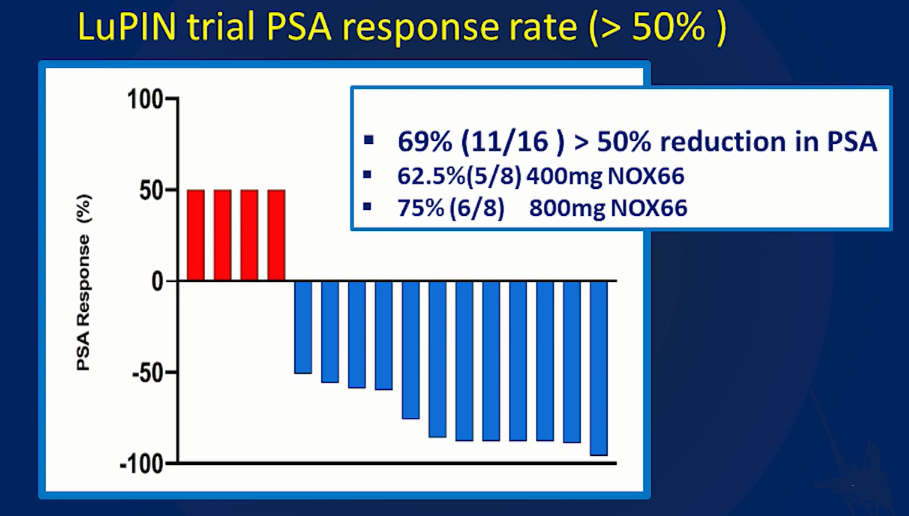 LuPIN Trial PSA Response Rate (Source: St Vincentâs LuPIN trial Presentation SNMMI 2019)
LuPIN Trial PSA Response Rate (Source: St Vincentâs LuPIN trial Presentation SNMMI 2019)
Professor Emmett opened the presentation with an example of the patient who was injected with the 177Lu-PSMA-617 on its own and this showed a decent uptake but had eventually relapsed before he could reach the end of his treatment. The point that Professor Emmett tried to make was that the bigger problem is associated with the survival of the patients was to at least complete their full course of treatment, where the cancer is progressive and pain levels are high.
In this scenario, Professor Emmett explained that for biologically targeted radiotherapy be effective, the agents should involve number of factors like:
- Number of receptors expressed on cancer cell surface.
- Strength of peptide receptor binding.
- Power and range of the internalised radioactive decay.
- Radiation sensitivity of the cancer cell.
To address this, Noxopharm focuses on the radiation sensitivity of the cancer cell; that is, how the cancer cells of the body respond to the radiation therapy and the radiation reaching to every cancer cell around the patientâs body, when every other drug stopped working in the men with late-stage prostate cancer.
Under the ongoing LuPIN trial, the company used the combination of two experimental drugs that includes:
- Veyonda®: Noxopharmâs lead drug candidate Veyonda®, also known as NOX66, is a gene specific radiation sensitiser that targets cancer cells by blocking the catalytic activity of ENOX2 on the cancer cell surface. Idronoxil is the active compound in NOX66.
- 177Lu-PSMA-617: It is radiopharmaceutical licensed to US biotech company, Endocyte Inc., which was acquired late last year by Swiss Mega pharma company, Novartis, in a USD$2.1 billion deal.
Hypothesis of the Study: Professor Emmett stated that the hypothesis of the trial was: âUsing combined cancer targeted treatments leads to synergistic improvement in treatment response with minimal increase in side effects and overall improved quality of life for men with progressive metastatic castration-resistant prostate cancer (mCRPC).â
The hypothesis was based on a number of pre-clinical studies that Noxopharm performed with Veyonda® in combination with radiotherapy, one being in a mouse genotype study in a mammary tumour model.
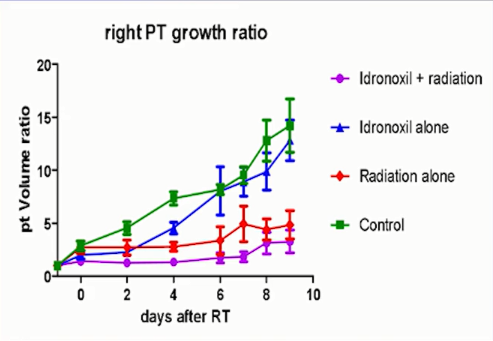
Tumour Growth Rate (Source: St Vincentâs LuPIN trial presentation SNMMI 2019)
Details of the Study: Under the 56-patient Phase Ib/IIa LuPIN study, the first 16 patients were divided into two cohorts of 8, who were reportedly given 6 injections over 8 months, at the interval of 6 weeks. Cohort 1 received 400 mg Veyonda® and Cohort 2 received 800 mg Veyonda®.
The study is focused to evaluate the efficacy and safety of Veyonda® combined with the radiopharmaceutical, 177Lu-PSMA-617, in men with progressive metastatic castration-resistant prostate cancer (mCRPC) following cabazitaxel, docetaxel, abiraterone and/or enzalutamide.
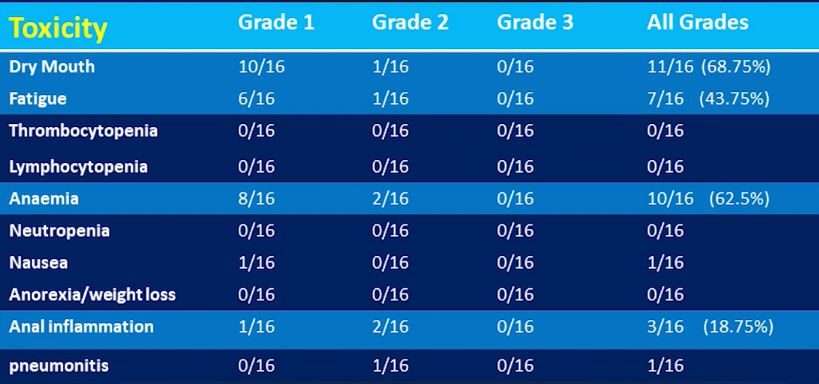 Toxicity Profile of Patients in the study (Source: St Vincentâs LuPIN trial presentation SNMMI 2019)
Toxicity Profile of Patients in the study (Source: St Vincentâs LuPIN trial presentation SNMMI 2019)
Professor Emmett informed that blood samples were taken fortnightly and number of questionnaires were taken from the patients for toxicity including pain, xerostomia and quality of life. The most common symptom observed in the 16 patients was the dry mouth. It was reportedly observed that anal inflammation occurred in 3 patients particularly within the 800mg dose.
Results of the Study:
Survival Rate: Overall survival in this LuPIN trial so far was 100 per cent at 3 months and 93 per cent at 6 months and at the time of reporting was 81 per cent after a median follow-up duration of a year. This outlines a median PSA Progression Free Survival (PFS) of 8.4 months in this trial at the moment. Overall survival is not yet reached.
The conference heard that 56% (9 out of 16 men) were able to complete their full treatment course without any relapse, an encouraging increase on other trials. Further, 44 per cent (7 out of 16) progressed before completing the full 6 cycles, with 25 per cent (4 out of 16) completing 3 cycles and 19 per cent (3 out of 16) completing 4-5 cycles.
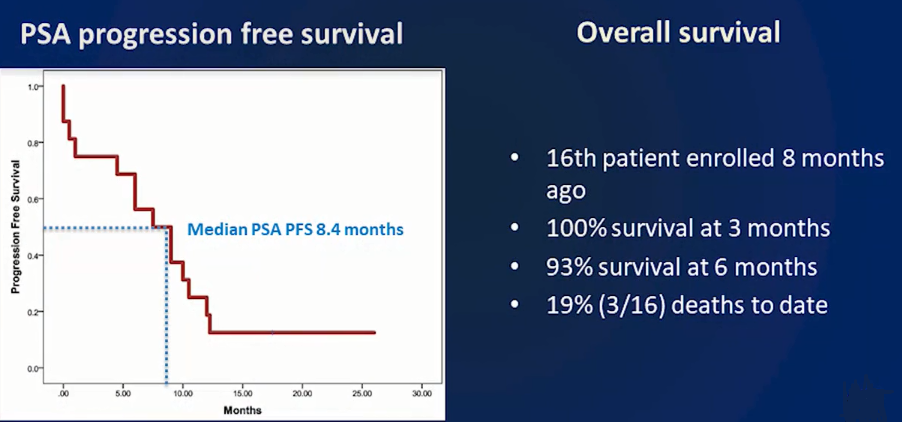 Source: St Vincentâs LuPIN trial Presentation SNMMI 2019
Source: St Vincentâs LuPIN trial Presentation SNMMI 2019
PSA Response Rate: The Prostate-specific antigen (PSA) response rate was 62.5% (Cohort 1) and 75% (Cohort 2), with an overall response rate of 69%.
Professor Emmett said, âThese initial results show that the combination treatment was well tolerated with early signs of efficacy.â Further, the study has now been expanded to include an additional 24 more patients.
As stated by Dr Graham Kelly, NOX Executive Chairman, the data complements the encouraging interim results reported from the Companyâs DARRT-1 study in May 2019, also involving patients with late-stage mCRPC, and offers further research indications for the immunological activities of Veyonda®.
NOX settled the dayâs trading session at A$0.480 on 5 July 2019 (12:21 PM AEST).
Also Read: Noxopharm â A Lens Over Its Clinical Trials For The Treatment Of Prostate Cancer
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. The above article is sponsored but NOT a solicitation or recommendation to buy, sell or hold the stock of the company (or companies) under discussion. We are neither licensed nor qualified to provide investment advice through this platform.






