Operating as a clinical-stage drug development company, Noxopharm Limited (ASX: NOX) believes it might achieve the potentially curative, or a possible long-term remission outcome, with its cancer treatment. It is engaged in the treatment of cancer, along with its Group subsidiary, Nyrada Inc (non-oncology) focusing on four pre-clinical programs in the areas of anti-neuroinflammation, neuroprotection, and anti-hypercholesterolaemia.
It has been scientifically posed that if cancer is due to the bodyâs defense systems being disabled, then re-enabling those defense systems is the most rationale way to fight cancer. With the drug candidate called Veyonda®, Noxopharm has stepped into this area of research to aim to develop a first-in-class activator of the innate immune system of the body. The company is seeking to develop the potential immuno-oncology and dual-acting cytotoxic drug candidate, Veyonda® to be used in combination with chemotherapy and radiotherapy for the treatment of prostate and other cancers, that can be likened to âworking with, not against the bodyâs defenses.â The drug compound Idronoxil is the active constituent in Veyonda®.
Drug Targeting Procedure (Source: Companyâs website)
In a recent interview to the media, CEO and CMO, Dr Greg van Wyk stated that âAt this stage, Noxopharm has two ongoing trials using Veyonda® in men with late-stage prostate cancer.â One combines Veyonda® with an injectable form of a radiopharmaceutical (LuPIN-1) and the other combining Veyonda® with the use of external beam low dose radiotherapy (DARRT-1).
Below image depicts companyâs ongoing clinical programs in the oncology segment:
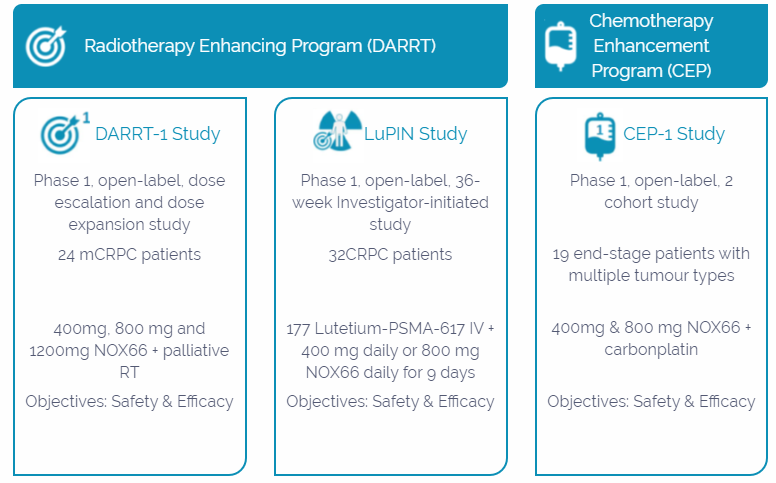 Details of Companyâs Clinical Programs (Source: Companyâs website)
Details of Companyâs Clinical Programs (Source: Companyâs website)
The company has recognised two fundamental clinical development objectives, to ensure maximisation of financial and clinical benefits:
- To establish Veyonda® as an essential adjunct to radiotherapy in the treatment of prostate cancer across multiple stages of the disease continuum.
- To discharge portfolio risk by studying Veyonda® in combination with radiotherapy and in combination with non-radiotherapy-based treatments.
Radio-enhancing programs: DARRT and LuPIN
DARRT stands for Direct and Abscopal Response to RadioTherapy. In this program, patients receive a short course of low dose external beam radiotherapy and Veyonda®, aimed to instigate an anti-cancer effect, in which there has been evidence of a reduction of pain in some patients - a major issue in late-stage prostate cancer.
The study has recently shown long-lasting responses to the single 15-day treatment producing effective anti-cancer response rate over a period of at least six months. In some cases, patients have achieved complete pain relief by the use of Veyonda® coupled with low-dose radiotheraphy.
Under the LuPIN study, the company tests Veyonda® for its ability to increase incidence, degree, and durability of a response to radioligand therapy (Lutetium-PSMA-617) that targets prostate-specific membrane antigen (PMSA) on the surface of prostate cancer.
Both trial interim results to date are hoping to show encouraging potential in Veyonda® to become a standard co-treatment with two forms of radiotherapy in safe and well-tolerated way.
Chemotherapy Enhancement Program
Under Chemotherapy Enhancement Program (CEP), Noxopharm evaluates Veyonda® for its efficacy when combined with one kind of standard chemotherapy in some advanced solid cancers (end-stage, metastatic).
Noxopharm recently announced an expansion of Veyonda® CEP Program based on its Phase 1b CEP-1 study, which confirmed safety and good tolerability with encouraging efficacy in late-stage cancers. This study demonstrated some efficacy signals for the combination of Veyonda® and carboplatin, including:
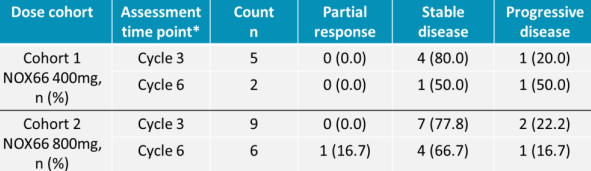
Phase 1b CEP-1 study Results (Source: Companyâs website)
Together these programs aim to cement Veyonda® as a key, versatile treatment in the lucrative (multi-billion-dollar) cancer market.
Stock Performance:
NOX stock has climbed up by 20% today, trading at $0.660 (As at 1:35 PM AEST, 20 May 2019). The stock has returned an attractive 197.30% return since its listing on ASX in August 2016, with impressive YTD return of 34.15%.
Also Read: Looking Through The Business Model Of This Drug Development Company â Noxopharm
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. The above article is sponsored but NOT a solicitation or recommendation to buy, sell or hold the stock of the company (or companies) under discussion. We are neither licensed nor qualified to provide investment advice through this platform.



