Highlights
- Prescient presented CellPryme-A data at the CAR-TCR Summit in Boston, MA, the largest CAR-TCR meeting in the world
- CellPryme-A deals with the aggressive tumour microenvironment, improves the penetration of CAR-T cells into tumours, demonstrates good synergy with CellPryme-M and increases the expansion of CAR-T cells in-vivo.
- As per PTX, the new data on CellPryme-A provides a partnership opportunity for the company with external parties and potential commercialisation
- CellPryme-A is ready for clinical testing and can be incorporated into clinical studies of existing cell therapies
Prescient Therapeutics (ASX:PTX) has presented exciting new data on CellPryme-A at the 7th Annual CAR-TCR Summit in Boston, MA. The summit is one of the world’s leading forums in the CAR and TCR fields of cellular immunotherapy.
What is CellPryme-A?
CellPryme-A is an adjuvant/neoadjuvant developed by Prescient in collaboration with Peter MacCallum Cancer Centre (Peter Mac). It is administered to cancer patients as an intravenous infusion before or shortly after starting cellular immunotherapy Chimeric Antigen Receptor (CAR)-T cell therapy. This idea behind CellPryme-A administration is to deal with the aggressive tumour microenvironment (TME).
TME is known to inhibit the tumour-killing ability of CAR-T cells and similar kinds of cellular therapy.
Exciting new data
CellPryme-A deals with TME by lowering the number (around 66%) of oppressive regulatory T cells (Tregs). These T cells around solid tumours counteract the efficiency of CAR-T and other cancer therapies and are some of the most immunosuppressive immune cells in the body.
As per PTX, during preclinical studies, CellPryme-A presented superior tumour killing and survival. However, it had greater effects when combined with CellPryme-M. CellPryme-M is the CAR-T manufacturing technology of PTX.
The new data on CellPryme-A provides another partnership opportunity for PTX with external parties and potential commercialisation. The technology is ready for clinical testing and can be incorporated into clinical studies of existing cell therapies.
In addition to lowering the number of Tregs, CellPryme-A offers the following benefits in cancer treatment:
- It substantially enhances CAR-T cell penetration into tumours.
- It demonstrates good synergy with CellPryme-M. In an aggressive MC38 colon cancer model, the addition of CellPryme-A to CellPryme-M pre-treated CAR-T cells revealed remarkable synergies.
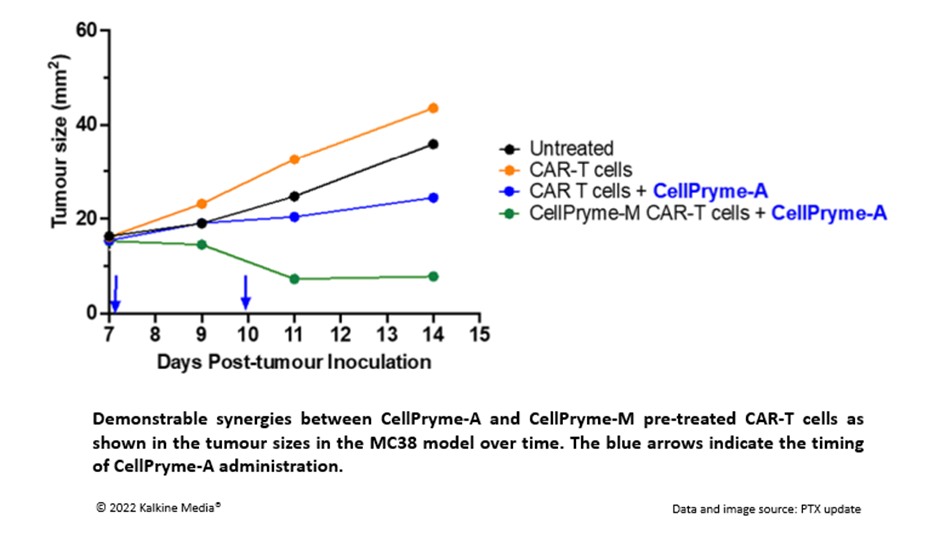
- The combination of CellPryme-M pre-treated CAR-T cells and CellPryme-A also enhanced the expected survival of treated animals compared to the application of conventionally manufactured CAR-T cells.
- CellPryme-A dramatically increases the expansion of CAR-T cells in vivo. It was evident when the addition of CellPryme-A to CellPryme-M pre-treated CAR-T cells resulted in over six-fold growth of CD4+ CAR-T cells and more than a nine-fold expansion of CD8+ CAR-T cells.
Commenting on the new data, Prescient’s Senior Vice President of Scientific Affairs, Dr Rebecca Lim said, “Synergies between CellPryme-A and our CellPryme-M platform give cellular immunotherapy the best chance at success based on these animal studies.”

Bottom line
Data reveals that CellPryme-A and CellPryme-M can work synergistically to deliver an unparalleled potency in solid cancer with even conventional CAR-T cells.
At the time of writing this article (23 September, 11:20 AM AEST), shares of PTX were trading at AU$0.187 with a gain of 1.351%.



