Highlights
- Prescient Therapeutics (ASX:PTX) has updated on continued promising results in PTX-100 T Cell Lymphoma Phase 1b Cohort.
- The latest data has exhibited two new complete responses in the patients since the last update.
- The results indicate that 7 of 10 evaluable patients had durations of response exceeding standard of care.
- The compound continues to exhibit an excellent safety profile, the company says.
- The company plans to proceed expeditiously to a registration trial.
Prescient Therapeutics Limited (ASX:PTX) has shared an encouraging update for the ongoing clinical trial of its PTX-100 compound in patients with relapsed and refractory T cell lymphomas (TCL).
The compound, which is under the Phase 1b expansion cohort, continues to indicate encouraging clinical activity, with two patients showing complete responses (complete eradication of cancer) since the last update in November 2022. The company says that this observation is not generally expected in this disease (relapsed and refractory peripheral TCL).
Also, the latest update suggests that the compound has shown an excellent safety profile at the highest dose of 2000 mg/m2.

Excellent safety profile and encouraging clinical activity
Under the study at Epworth Hospital in Melbourne, 13 TCL patients received PTX-100 dosing including five patients with cutaneous TCL (CTCL) and eight patients with PTCL.
Safety - The clinical-stage oncology company suggests that the compound continues to display an excellent safety profile on the study.
Neutropenia, thrombocytopenia and anaemia are among the few of the adverse events observed. The adverse events were observed in the same patient; however, they recovered/resolved these events.
It is to be noted that such side effects are not uncommon in treating this patient population and are likely manageable, says the company.
Encouraging clinical activity - PTX-100 continues to exhibit encouraging clinical activity in the difficult-to-treat patient population, especially when considered against responses expected from current standards of care.
The data suggests that 7 of 10 evaluable patients had durations of response exceeding standard of care.
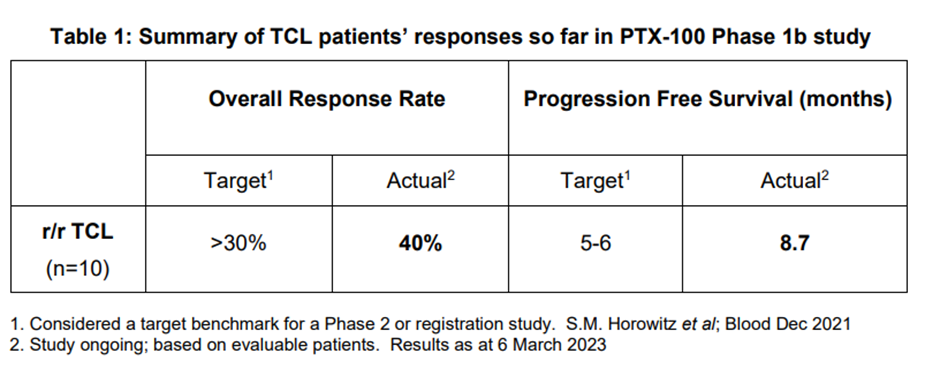
Image source: PTX update
Prescient to apply for FDA meeting by end-2023
The company recently secured the additional Orphan Drug Designation from the US FDA for PTX-100. Prescient has been suggested by regulatory advisors to recruit seven additional patients, to get more data for a more meaningful and productive dialogue with the US FDA.
Given satisfactory Phase 1b study results, the company intends to undertake a subsequent Phase 2 trial in TCL.

Data source: PTX update
Also, the company plans to sought clarification on the dose optimisation and dose schedule considerations for the Phase 2 study pursuant to the FDA’s Project Optimus. This project targets maximising drug efficacy along with its tolerability and safety.
Later in 2023, the company will be filling an application for a meeting with the FDA. If successful, the company expects to open the registrational Phase 2 study within a period of one year.
Prescient is already working on planning towards undertaking another manufacturing campaign of PTX100 in order to facilitate further studies. The company will conduct and document the manufacturing process at higher levels of rigour required to support later stage trials and regulatory submissions.
PTX shares were trading at AU$0.120 midday on 16 March 2023.




