Highlights
- Invion has secured positive screening results from the in vitro study of PhotosoftTM technology against the Zika virus.
- Currently, there is no treatment available for the Zika virus, and its market is estimated to be worth $25bn and will grow at a CAGR of 5.4% through 2027.
- The study suggests that selected PhotosoftTM compounds are more effective and safer against the virus than the control (benchmark) antibiotic Monensin.
- This is the first set of test results on infectious diseases, and it comes at a time when the world is on heighten alert to the next pandemic.
Invion Limited (ASX:IVX) has set its first foot forward in the multi-billion Zika virus market with excellent preliminary results from in vitro studies of the PhotosoftTM technology. The study results highlighted that selected PhotosoftTM compounds are effective against the virus and more efficacious than Monensin.
This is the first set of studies using PhotosoftTM compounds on infectious diseases. The development presents potential for the technology in a period where the next global pandemic could be waiting around the corner, said IVX Chairman and CEO Thian Chew.
PhotosoftTM is a novel next-generation Photodynamic Therapy (PDT) currently under development by Invion.
IVX tapping the multi-billion-dollar industry
Zika has been reported in 86 countries, particularly in Asia and Africa. Currently, no treatment is available for Zika virus infection or its associated diseases. The estimated market size of the Zika virus is worth US$16.98 billion (AU$25 billion) in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 5.4% to 2027, as per the company update referring to a report by Market Data Forecast.
Further, a recent report titled “Strengthening Australia’s Pandemic Preparedness” by CSIRO highlighted the development of novel antivirals as a major area of science and technology for bolstering the pandemic preparedness of the country.

Symptoms of the Zika virus, mainly transmitted by the Aedes Aegypti mosquito, include headache, joint pain, and skin rash. The virus has been linked to birth defects and other neurological complications.
Summary of results from the study
The study of PhotosoftTM against the virus was conducted by Viroclinics-DDL and Virology Research Services. Viroclinics is a leading contract research and clinical laboratory service company.
The study used Zika Strain MP1751 (African lineage). The assessment was done using a quantitative assay to measure virus replication in the presence of increasing concentrations of the PhotosoftTM compounds and a control compound (Monensin) compared to replication in the absence of the product.
Monensin is known to have anti-Zika activity in in vitro tests, but its in vivo toxicity renders it unfit for use in humans.
Following are the main highlights of the results:
- On exposure to specific light wavelengths, selected PhotosoftTM compounds exhibited more than 99% inhibition, i.e., antiviral activity against Zika
- Cytotoxicity levels were also lower
- PhotosoftTM compounds had more than 190 times higher therapeutic index than Monensin
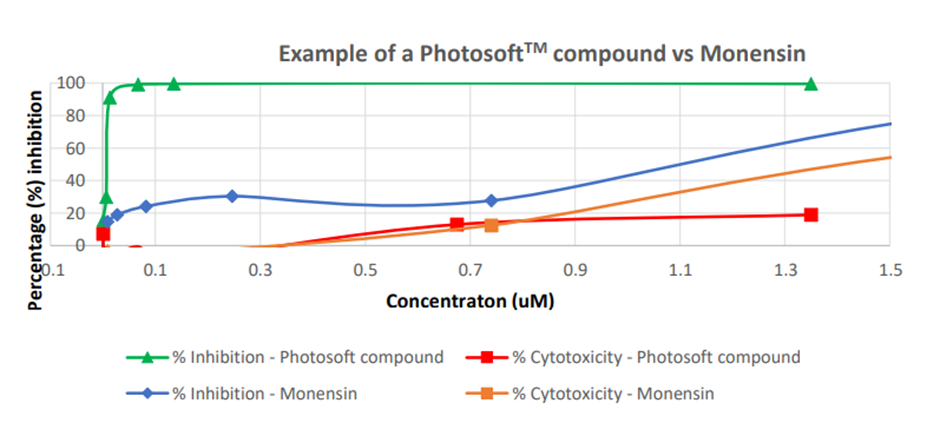
Image source: IVX update
Image description: Photosoft versus Monensin
To sum up, the studies demonstrated that selected PhotosoftTM compounds are effective against the virus. They were also more efficient than the control (benchmark) antibiotic Monensin.
At the time of writing this article, share price of Invion was AU$0.013 apiece on 8 September 2022.



