Highlights
- Invion has reported positive screening results from in vitro studies of 10 PhotosoftTM compounds against the dengue virus.
- Monensin, an antibiotic, was used as a control in the study.
- Eight out of the 10 compounds showed Therapeutic Indexes superior to Monensin's.
- Several of the PhotosoftTM compounds demonstrated a higher safety profile than Monensin’s.
Shares of Invion Limited (ASX:IVX) are trading on the ASX today at AU$0.010 a piece with an upsurge of 5%. The company developing Photodynamic Therapy has reported positive screening results from in vitro studies of PhotosoftTM against the dengue virus.
Invion, a life sciences company, claims to be leading the global research and development of PhotosoftTM technology for treating a range of cancers, atherosclerosis, and infectious diseases.
Dengue is transmitted through the bite of an infected Aedes aegypti mosquito. It causes intense pain in joints and muscles, from where it got its nickname, ‘breakbone fever’. The global treatment market of dengue is projected to reach US$1.3 billion by 2030, which equals a compound annual growth rate of 11.2%.
These in vitro studies on dengue are the third set of successful, early-stage tests undertaken by Invion on enveloped viruses. An enveloped virus has a lipid bilayer membrane on its outer part.
The other two studies in which PhotosoftTM showed antiviral activity were based on the Zika virus and SARS-CoV-2 (Delta and Omicron variants) viruses.
Invion is conducting these tests as part of testing on infectious diseases using PhotosoftTM technology. Results from these as well as other studies will help the company decide which infectious disease target(s) holds the greatest potential to advance to human applications.
PhotosoftTM versus Monensin against dengue
Tests were conducted on 10 PhotosoftTM photodynamic compounds at eight concentrations. Monensin, an antibiotic with known activity against the dengue virus, was used as a control.
The results showed that eight of the 10 compounds displayed antiviral activity against the dengue virus after exposure to a specific wavelength of light (660nm). In the absence of this light source, none of the compounds in the study were antiviral.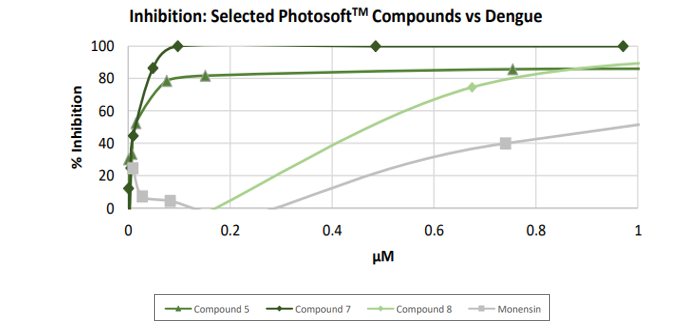
Image source: Company update
Image description: The percentage inhibition of the dengue virus against the logarithm of compound concentration in µM. Data included after LED exposure.
For the eight PhotosoftTM compounds that showed antiviral activity, the EC50 (half maximal effective concentration) values ranged between 11.1nM and 539nM. These values were considerably lower than those of Monensin (1031nM).
Furthermore, the test compounds had low cytotoxicity against assay cells. This coupled with lower effective concentrations resulted in higher Therapeutic Indexes (TI = CC50/EC50) than Monensin’s, implying that several PhotosoftTM compounds had a higher safety potential.
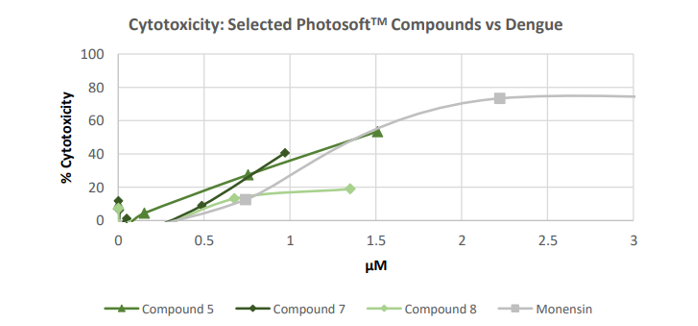
Image source: Company update
Image description: The percentage of cytotoxicity of Vero E6 cells (assay cells) against the logarithm of compound concentration in µM. Data included for after LED exposure
Moreover, the two most potent anti-dengue compounds, Compound 7 and Compound 1, exhibited low cytotoxicity to establish CC50 (the concentration of the 50% cytotoxic effect) values. Both compounds showed less than 48% cytotoxicity at the highest tested concentration, with little-to-no cytotoxicity at lower concentrations.
Contrary to this, Monensin exhibited antiviral activity against dengue and demonstrated significant cytotoxicity, after light exposure and when left unexposed.
The following image shows the EC50, CC50, and TI values against the dengue virus after LED exposure or when maintained unexposed:
Image source: Company update