Highlights
- Imugene’s VAXINIA MAST trial has cleared cohort 3 IT monotherapy dose escalation study.
- With this development, Imugene can recruit for IT cohort 4 of the monotherapy dose escalation.
- In cohort 4, VAXINA will be administered in 3-6 patients.
Australian clinical-stage biotechnology company Imugene Limited (ASX: IMU) informed that the phase 1 VAXINIA MAST (metastatic advanced solid tumours) trial has cleared the cohort 3 intratumoral (IT) monotherapy dose escalation study.
The trial is designed to assess the safety of novel cancer-killing virus CF33-hNIS (VAXINIA).
With this development, Imugene can begin with recruitment for the cohort 4 IT arm of the monotherapy dose escalation. In this study, VAXINIA will be administered in 3-6 patients.
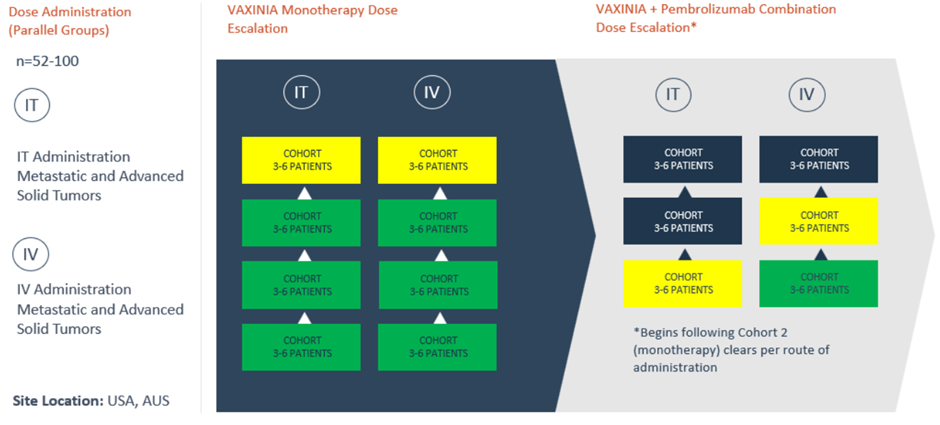
Image source: Company update
Shares jump
Triggered by the update, the company’s shares jumped nearly 3% to trade at AU$0.091 at the time of writing on 14 August 2023.
Here is what Imugene’s MD & CEO Leslie Chong commented on the latest development

Image source: Company update
Details of the trial
The Phase 1 MAST trial started by administering a low dose of VAXINIA to patients suffering from advanced or metastatic solid tumours and who have undergone through a minimum of two prior lines of standard of care treatment.
The study is designed to recruit a maximum of 100 patients across around ten trial sites in Australia and the United States.
The oncolytic virus, developed by the City of Hope, has been shown to shrink pancreatic, ovarian, breast, lung and colon cancer tumours in animal models and preclinical laboratory.
The title of the clinical trial is:

Data source: Company update
The study, which began in May 2022, is expected to run for nearly two years. The trial is being funded via existing resources and budgets.






