Highlights
- Imugene has dosed the first patient as part of intravenous (IV) cohort 2 of the Phase 1 MAST study
- The study is evaluating the safety of the novel cancer-killing virus CF33-hNIS (VAXINIA)
- Also, IMU presented positive new data from overall survival results in its HER-Vaxx HERIZON study at the ESMO Asia Congress 2022 in Singapore
One of the leading clinical-stage immuno-oncology companies, Imugene Limited (ASX:IMU), today announced that the first patient had been dosed as part of intravenous (IV) cohort 2 of the Phase 1 MAST (metastatic advanced solid tumours) study. The trial is being performed to measure the safety of the cancer-killing virus CF33-hNIS (VAXINIA).
Also, Imugene presented positive new data regarding overall survival results in its HER-Vaxx HERIZON study at the ESMO Asia Congress 2022 on Sunday, 4 December 2022, in Singapore.
Following upbeat announcements, IMU’s shares rallied by more than 5% today and were trading at AU$0.195 apiece at 10:57 AM AEDT.
IMU had announced earlier in November that its intravenous cohort 1 had been cleared, following which cohort 2 can begin. The intratumoral (IT) arm of the trial is also advancing after the first patient in cohort 2 was administered a dose on 27 October 2022.
About multicenter Phase 1 MAST trial
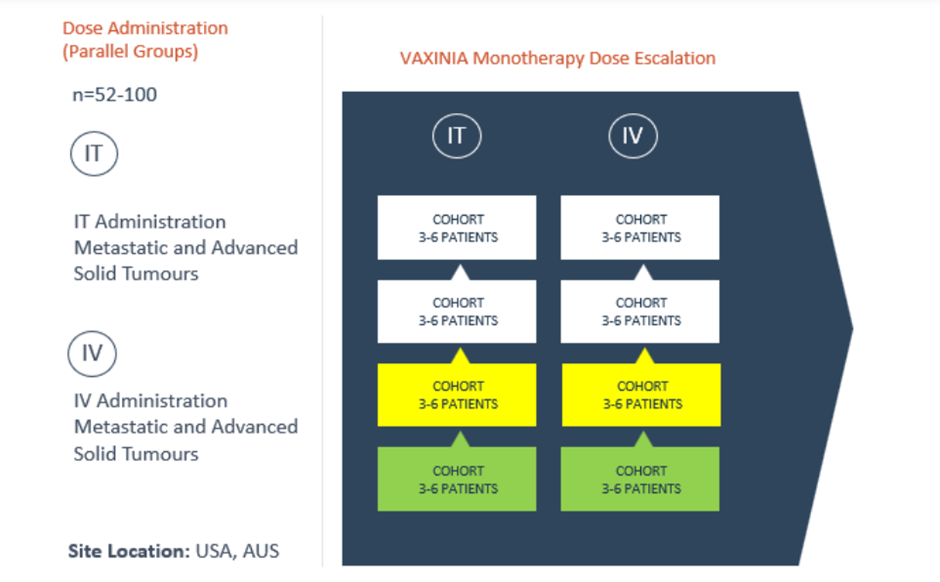 Image source: Company announcement
Image source: Company announcement
The Phase 1 MAST clinical trial is titled “A Phase I, Dose Escalation Safety and Tolerability Study of VAXINIA (CF33- hNIS), Administered Intratumorally or Intravenously as a Monotherapy or in Combination with Pembrolizumab in Adult Patients with Metastatic or Advanced Solid Tumours (MAST).”
In the multicenter Phase 1 MAST trial, a low dose of VAXINIA was delivered to patients with metastatic or advanced solid tumours with minimum of two prior lines of the standard of care treatment. The oncolytic virus developed by City of Hope has demonstrated to reduce breast, ovarian, colon, lung, and pancreatic cancer tumours in a preclinical laboratory and animal models.
After the enrolled subjects in the monotherapy group get treated with the lowest doses of VAXINIA and acceptable safety has been observed, new patients will be treated with a combination of CF33-hNIS and immunotherapy pembrolizumab. The combination treatment is likely to commence once Cohort 2 is cleared as per the route of administration.
Up to 100 patients will be recruited for the study across 10 trial sites in Australia and the United States. The company expects to complete the study in about two years.
Management commentary
 Image: © 2022 Kalkine Media®, Data: Company Announcement
Image: © 2022 Kalkine Media®, Data: Company Announcement
IMU featured at ESMO Asia Congress 2022
Imugene’s oral presentation and abstract about positive new data from overall survival results in its HER-Vaxx HERIZON study were presented at the ESMO Asia Congress 2022 under the title 'HERIZON Overall Survival Results: A study of IMU-131, a HER2 Targeting Peptide Vaccine, Plus Standard of Care Chemotherapy in Patients with HER2 Overexpressing Metastatic or Advanced Gastric/GEJ Cancer'.
Principal investigator Marina Maglakelidze at the event talked about the study design, the demographics and characteristics of the 36 subjects who participated in the study, and information about safety and adverse occurrences.
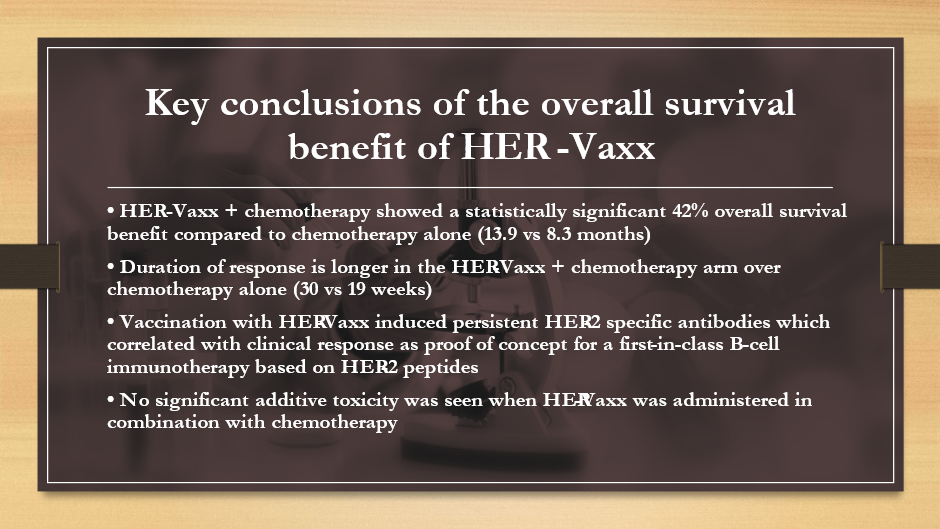 Image: © 2022 Kalkine Media®, Data: Company Announcement
Image: © 2022 Kalkine Media®, Data: Company Announcement
You may see the complete presentation here.






