Highlights
- CHM 1101, a CAR T therapy, is being designed to address high unmet medical needs of people with recurrent or progressive glioblastoma.
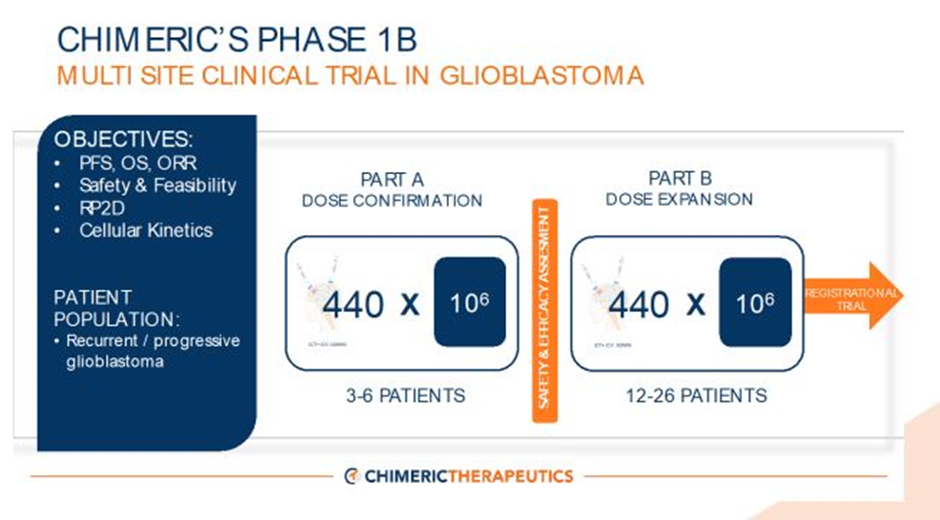
- The Phase 1B trial's Part A at the City of Hope Cancer Centre would treat three to six patients.
- Phase 1B trial of CHM 1101, now approved by the ethics review board, would see participation of multiple clinical sites.
Chimeric Therapeutics (ASX: CHM), working on cell therapies like CAR T for cancer treatment, has informed that its CHM 1101 clinical trial (Phase 1B) has received approval from ethics review board. The approval is for the initiation of a multi-site Phase 1B trial of the therapy in people with recurrent and/ or progressive glioblastoma multiforme.
CHM would undertake the evaluation of clinical safety and efficacy data from the cell therapy’s Phase 1 dose escalation/ confirmation cohort by end of this year.
Part A of the trial will treat 3-6 patients. CHM has also confirmed that Part B of the trial design would begin once the results of the assessment of data support further development.

Data source: CHM ASX announcement dated 26 April 2023; Image source: Pixabay.com
The development
The listed cell therapy company has received ethics approval for CHM 1101 multi-site clinical trial (Phase 1B), which would further push the trial of the “first in class” CAR T therapy in patients with recurrent and/ or progressive glioblastoma multiforme. Chimeric has said that this recent development would allow for the two-part Phase 1B study in which patients would be enrolled at multiple clinical trial sites.
Chimeric Therapeutics would provide more details on the Phase 1B trial design and goals in June this year at the American Society of Clinical Oncology annual meeting.
The Part A trial would involve treatment of up to six patients, which would complete the Phase 1 dose escalation/ confirmation study. At the later stage, there would be a Part B dose expansion cohort with enrolment of 12 to 26 patients and use of the recommended Phase 2 dosing plan. Chimeric states that after this, the registration trial of CHM 1101 will start in alignment with regulatory feedback.
Source: CHM ASX announcement dated 26 April 2023
About Chimeric
Chimeric Therapeutics' portfolio includes two autologous CAR T cell therapies (CHM 1101, and CHM 2101) and an allogeneic NK cell therapy platform (CHM 0201). The ASX-listed company has two ongoing clinical trials and planning is underway to begin more clinical programs this year.
The company's shares on ASX traded at AU$0.071, up over 5.9%, at the time of writing on 26 April 2023, with market cap of over AU$29 million.



