Medical devices are one of an essential parts of health care system. These are used for accurate diagnosis of the disease and help health care professional to treat patients and improve their life quality. Medical device market in Australia has already reached in its mature stage with a well-formed regulatory system. Australia imports about 80% of the medical devices whereas the domestic production is controlled by subsidiaries of global companies.
In the Australian health care system, Therapeutic good administration (TGA) is responsible for regulating therapeutic good such as vaccines, medical devices, medicine, and others. TGA is a part of the Australian Government Department of Health.
Medical Devices Categories in Australia- Section 41BD of the Therapeutic Goods Act 1989 defines medical devices and implants in Australia.
Classification of medical devices as per TGA-
| Classification | Level of risk |
| Class I | Low |
| Class I - supplied sterile Class I - with a measuring function Class II | Low-medium |
| Class IIb | Medium-High |
| Class III | High |
| Active Implantable medical devices (AMD) | High |
Letâs discuss few health care stocks which are engaged in manufacturing medical devices and implants- RESONANCE HEALTH LIMITED, ALLEGRA ORTHOPAEDIC, IMAGION BIOSYSTEMS
Resonance Health Limited (ASX: RHT)
Australian headquartered health care company Resonance Health Limited (ASX: RHT) which is engaged in developing and delivering non-invasive medical imaging software and services. Many Clinicians and pharmaceutical companies use the Company's products.
Currently, Resonance has the following products in the market-
- FerriSmart- Itâs the first regulatory approved artificial intelligence (AI) solution in the world for the quantification of liver iron concentration (LIC). FerriSmart is the only medical device for determination of LIC which has received approval from TGA, CE Mark, and FDA.
- FerriScan®â FerriScan® was approved by the Food Drug and Administration (FDA) in 2005 for measurement of liver iron concentration (LIC). It is globally recognised as the gold standard for the quantitative analysis of LIC; based on non-invasive, MRI-based technology.
- Cardiac T2* with FerriScan®- Cardiac T2* is for detection of iron; additionally, it also provides binary analysis package with FerriScan and gives detailed information of body iron stores.
- HepaFat-Scan®- HepaFat-Scan® gained regulatory approval for the volumetric liver fat fraction (VLFF) in the US, Europe and Australia.
- Bone Marrow R2-MRI- It is an MRI-based product which is used for assessing bone marrow iron levels.
Source: Companyâs Report
On 28 October 2019 company released its quarterly report for the period ended 30 September 2019.
Key highlights for last quarter-
- In September 2019 quarter the cash receipts from customers were $891K, which is increased by 31% from the previous year September quarter.
- There is an increase in the total revenue for the September quarter, which was approximately $956K and the revenue is increased by 8% and 12% as compared to this year June quarter and last year September quarter respectively.
- Cash on hand of the company was $3.25m as of 30 September 2019, which is increased by $171K from June quarter 2019.
- Resonance has signed a new contract which is related to provide products and services to a pharmaceutical company for the clinical trial.
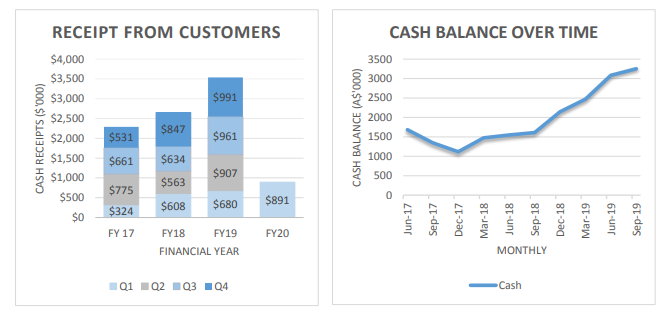
Source: Company Report
Annual General Meeting
Resonance would conduct an AGM on 28 November 2019 in UWA Water Sports Complex, Crawley.
According to an ASX announcement the company has updated its security trading policies. The updated policies are for both sale and purchase of any securities of Resonance. The changes are effective from 22 October 2019.
Stock performance-
The companyâs stock ended 31 October 2019 at $0.125, up by 4.167% with a market cap of approximately $51.26 million.
Imagion Biosystems Limited (ASX: IBX)
Imagion is an ASX listed company which is engaged in developing diagnostic imaging technology which is safe and non-radioactive. The company helps in the detection of cancer and other diseases by combining biotechnology and nanotechnology. The companyâs new technology MagSenseâ¢, which is a bio-imaging detection technology, uses disease-specific targeting nanoparticles and highly sensitive detectors to locate tumours and other infected cells by their molecular signature.
On 28 October 2019, the company announced a renounceable pro-rata offer of approximately 327,369,384 fully paid ordinary shares. The shares will be issued at $0.02 per share along with 1 attaching option. This will lead to a minimum subscription of $2 million and maximum subscription of $6.5 million.
Scientific Advisory Board
On 14 October 2019 company announced the addition of Dr John Hazle as the Chairman to its newly formed Scientific Advisory Board.
First-in-human study progress update
On 2 October 2019 Imagion provided an update on its progress and capital management for the first-in-human study. The company expects to start the first-in-human study for HER2 metastatic breast cancer test in the 2020 first half.
The company would focus on the following three main areas for achieving a successful First-in-human study for HER2 metastatic breast cancer-
- In obtaining the Regulatory approval needed to commence the study.
- Finalizing the clinical study plan and site selection.
- Injectable nanoparticle formulation manufacturing for use in the study.
Stock Performance
The companyâs stock settled on 31 October 2019 at a price of $0.022, which is up 10% from its previous close, having a market cap of nearly 6.55 million. The stock has a 52 weeks high price of $0.075 and a 52 weeks low price of $ 0.016.
Allegra Orthopaedics Limited (ASX: AMT)
An Australian based company Allegra Orthopaedics Limited (ASX:AMT) was established in 1994 and is engaged in providing orthopaedic products.
The company is developing orthopaedic products for both upper and lower limb.
- For upper limb, implants are available for Clavicle, shoulder and hand & wrist.
- Clavicle- Clavicle Fixation System (CFS),
- Shoulder- Arthrosurface HemiCAP® Shoulder and Arthrosurface NanoFX® (Nanofracture)
- Hand & wrist- KLS Martin IXOS® Distal Radius System
- For lower limb implants are available for Hip, knees and foot & ankle
Stock Information-
The companyâs stock settled at a price of $0.150 on 31 October 2019 having a market cap of nearly $14.93 million. The stock has a 52 weeks high price of $ 0.220 and a 52 weeks low price of $0.079.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. Kalkine Media does not in any way endorse or recommend individuals, products or services that may be discussed on this site. Our publications are NOT a solicitation or recommendation to buy, sell or hold. We are neither licensed nor qualified to provide investment advice.




