Botanix Pharmaceuticals Limited (ASX: BOT) is an Australian-headquartered company, engaged in the development of next-gen therapeutics that are safe and effective for the treatment of serious skin diseases. It is a clinical stage cannabinoid company whose pharmaceutical products contain a synthetic form of cannabidiol. Recently, Botanix Pharmaceuticals has also appointed its new President and Executive Chairman of the Board, Mr Vince Ippolito.
BTX has generated a tremendous return of 256.16 per cent on a YTD basis. Botanix Pharmaceuticals is planning to raise $40 million through the placement of ~190 million new shares. As reported by a leading media player, the company has also chosen two brokers - Argonaut and Bell Potter Securities - to assist it in searching for potential investors.
Let us take a deep dive into this clinical stage cannabinoid company:
Botanix Pharmaâs Product Portfolio
The products developed by the company are well supported by science and randomised clinical trials. The companyâs product pipeline at different stages of trials includes - BTX 1503, BTX 1308, BTX 1204 and BTX 1801- that are focussed on the treatment of several skin diseases like atopic dermatitis, plaque psoriasis and serious acne.
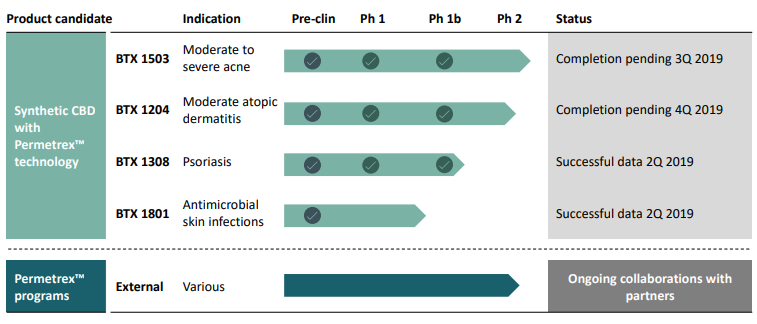
Source: Companyâs Report (20th June 2019)
Let us know more about each of these products below:
BTX1503
BTX 1503 is a safe and well-tolerated topical treatment that potentially addresses all of the three causes of acne. It is a transdermal gel formulation which is generally used to treat severe acne problem in teenagers and adults. As per the company, Acne is the most common skin disorder, which is impacting ~50 mn US residents. It is also a major cause of dermatologist visits and psychological difficulties in adolescents. The company believes that BTX 1503 has the potential to lower the severity or occurrence of serious acne, and it could also become an important alternative to some prevailing therapies associated with serious side effects.
BTX 1503 has successfully completed the Phase 1b clinical trial and is under Phase 2 trial currently. The company mentioned in its recently released Investor presentation that the four-week Phase 1b study data of BTX 1503 showed a marked reduction in inflammatory lesions and no serious adverse events.
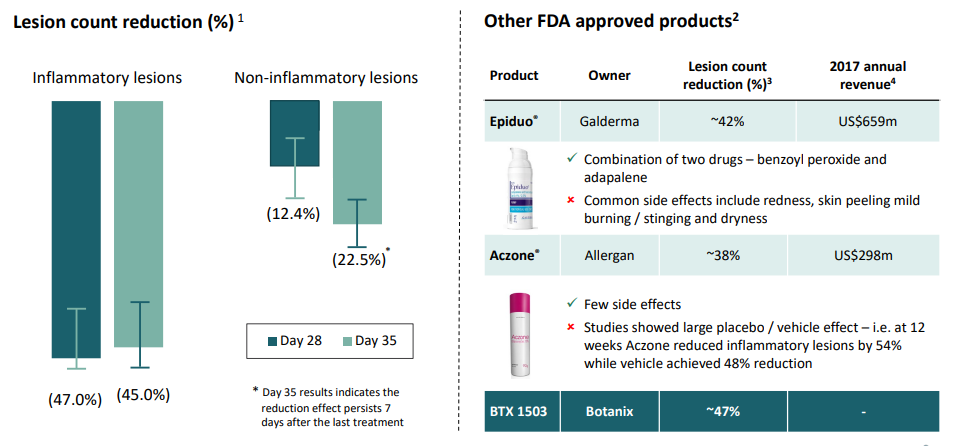
Phase 1b patient data for BTX 1503, Source: Companyâs Presentation (20th June 2019)
The Phase 2 study of BTX 1503 is a twelve-week randomised, double-blind, vehicle-controlled study which is being conducted to evaluate the safety and efficacy of BTX 1503 in patients with moderate to severe acne. The recruitment has been completed in Phase 2 study, with final subjects completing the in-life phase of the study in Q3 2019.
BTX 1308
It is a transdermal gel formulation which is generally used to treat serious plaque psoriasis that affects the autoimmune system of the patients. As per the company, BTX 1308 has the potential to lower the severity or occurrence of psoriasis lesions, and it could also lower keratinocyte proliferation. The company has recently completed the Phase 1b study of BTX 1308 and announced positive interim data in June 2019.
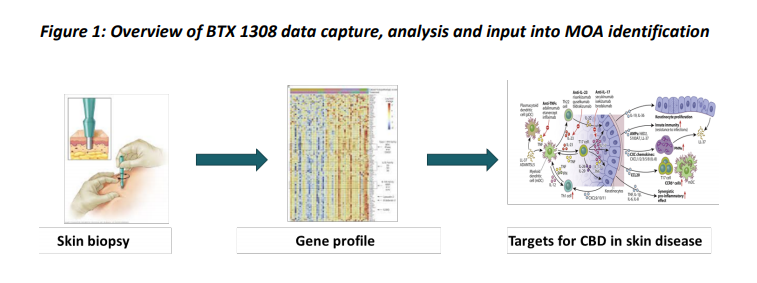
Source: Companyâs Report (19th June 2019)
The Phase 1b study tested BTX 1308 in a model that allows biopsies to be collected and helps validate the mechanism of action of CBD in psoriasis. The study found that PermetrexTM (novel skin delivery technology) effectively delivered CBD to the skin Iayers that were involved in the pathogenesis of skin disease and activated significant alterations in immune response and inflammatory pathways.
BTX 1204
BTX 1204 is used for the treatment of inflammatory skin disease, atopic dermatitis, which is most commonly observed in children. BTX 1204 is currently undergoing Phase 2 patient study, which is likely to complete by 4th quarter of CY2019. The Phase 1b study results of BTX 1204 supported the efficacy and safety potential of the product. In Phase 1b study, BTX 1204 was found twice as effective as vehicle (with efficacy still increasing) and displayed substantial improvement in the key signs of atopic dermatitis. The Phase 2 study of BTX 1204 is a twelve-week randomised, double-blind, vehicle controlled study which is being conducted to evaluate the safety and efficacy of the product in patients with moderate atopic dermatitis (AD).
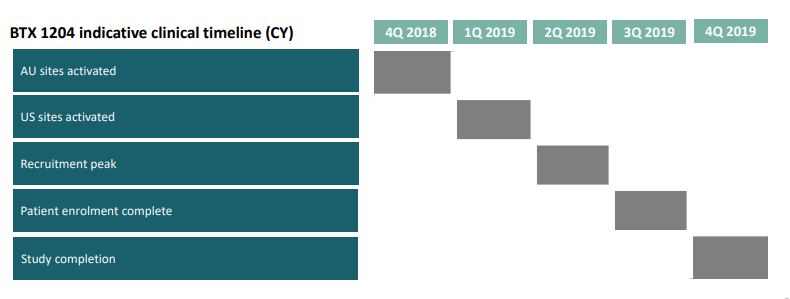
Source: Companyâs Presentation (20th June 2019)
BTX 1801
BTX 1801 is a first antibiotic that has formed non-resistance against superbugs. Recently, the company reported new data from studies conducted with its product BTX 1801. The studies highlighted that the natural potential of CBD was enhanced by the delivery of the product with Permetrex⢠technology. The studies also showed that BTX 1801 is effective against methicillin resistant Staphylococcus aureus and Staphylococcus aureus (âstaphââ), and a range of complex human and animal bacteria. BTX 1801 was found effective against biofilms, and it demonstrated efficacy in an independently tested animal model. Recently, the Botanix collaborator, Dr Mark Blaskovich, also presented the BTX 1801 studies results at the 2019 ASM (American Society for Microbiology) Microbe Conference.
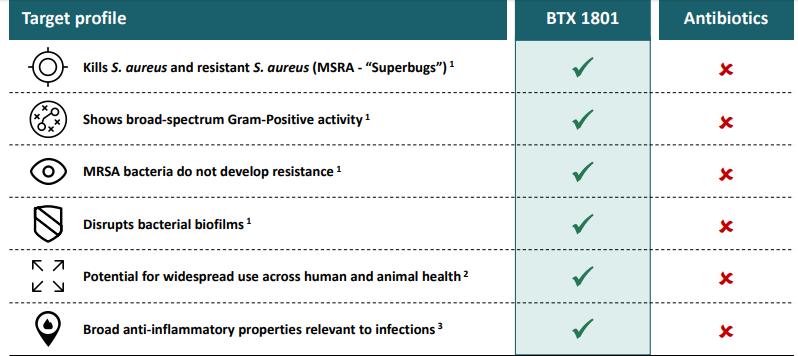
Source: Companyâs Presentation (20th June 2019)
The recently announced data from BTX 1801 and BTX 1308 studies has offered scientific support for Cannabidiolâs mechanism of action, which is extremely important to both Phase 2 atopic dermatitis and acne studies. Also, this new data has helped de-risk Phase 2 studies for BTX 1503 and BTX 1204, opening up a number of new opportunities in skin disease, antimicrobials and other new diseases.
In July this year, the company also announced new data from the recently undertaken studies around its new development program âAB 2367â. The results of the studies have shown that AB 2367 is as effective as currently used antibiotics at killing all the veterinary and human of strains C. difficile. The product was also found to be effective against ribotype 027 (super hypervirulent strain) and ribotype 078.
Financial Position of Botanix
As reported by the company in its March 2019 quarter report, Botanix had net cash outflows amounting to AUD 4.3 million with AUD 4.2 million invested in R&D activities during the quarter. The company held AUD 9.3 million at the end of the quarter. Also, it had estimated cash outflows of AUD 4 million for R&D activities for the June 2019 quarter.
Stock Performance Update
The shares of Botanix Pharmaceuticals Limited are currently placed in a trading halt, pending release of an announcement to the market. The shares are expected to begin their normal trading either on 1st August 2019 or when the announcement will be released to the market. BOT traded last on 29th July 2019 at AUD 0.260.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. Kalkine Media does not in any way endorse or recommend individuals, products or services that may be discussed on this site. Our publications are NOT a solicitation or recommendation to buy, sell or hold. We are neither licensed nor qualified to provide investment advice.






