Summary
- There are currently 180 COVID-19 vaccine candidates under development, according to the WHO.
- UK-based AstraZeneca said that its COVID-19 vaccine development is still on track despite the delay.
- On 12 September 2020, clinical trials of AstraZeneca/Oxford University’s COVID-19 vaccine candidate, AZD1222, resumed following a halt due to an adverse reaction in the UK after confirmation by the MHRA.
- Pfizer Inc and BioNTech are set to increase enrollment for their Phase 3 COVID-19 vaccine clinical trial to up to 44k volunteers.
- Both the companies anticipate that conclusive information on efficacy from Phase 3 trial is likely by October. The partners, last week, stated that they are targeting a regulatory filing in the US in October 2020.
The COVID-19 storm that has created havoc in the world, is not showing any signs of abating with the world witnessing an ongoing increase in the number of new cases daily. According to the WHO (World Health Organization), globally, there have been 28.92 million confirmed cases of COVID-19, including 922,252 fatalities as of 14 September 2020 (3:28 PM CEST).
Several healthcare companies are actively involved in COVID-19 vaccine development, seeking an answer to the quintessential question: Is there a cure for COVID-19?
In this article, we will discuss two vaccine developers that are in the late-stage clinical trial with their vaccine candidates and remained in the eye of market participants.
Before jumping to the two COVID-19 vaccine developers - AstraZeneca and Pfizer, let us first acquaint you in brief about the latest COVID-19 situation and vaccine contenders who are in the late stage of the clinical trial.
The WHO stated that 172 nations and several vaccine candidates are involved in COVID-19 vaccine Global Access Facility. At present, the equitable distribution of the COVID-19 vaccine is crucial for defeating the novel coronavirus and paving the path of recovery from the ongoing turmoil.
There are 180 COVID-19 vaccine candidates under development as per the draft landscape provided by the WHO on 9 September 2020. 35 out of the 180 candidates are in the clinical trial phases. The WHO is working in collaboration with researchers, global health organizations and business. Through the Access to COVID-19 Tools (ACT) Accelerator, the stakeholders intend to speed up the pandemic response.
Currently, nine companies/universities have progressed to late-stage clinical trials.
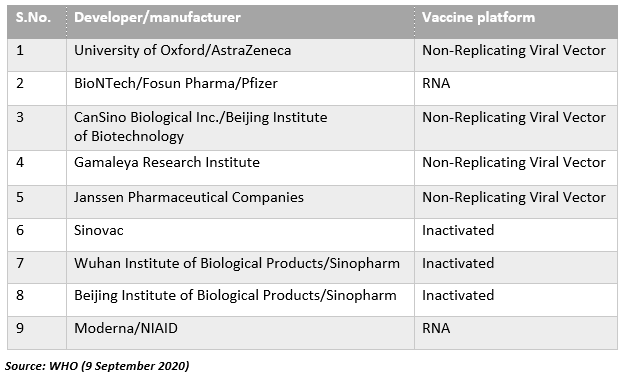
Let us now talk about COVID-19 vaccine development from AstraZeneca and Pfizer-
AstraZeneca’s COVID-19 Vaccine Development Still on Track Despite Delay
Oxford/AstraZeneca’s COVID-19 vaccine candidate, AZD1222, is one of the nine candidates across the world currently under Phase 3 clinical trials. The AZD1222 vaccine uses a weakened version of adenovirus, causing the common cold, engineered to code for the spike protein that the novel coronavirus uses to invade cells.
On 12 September 2020, clinical trials of AstraZeneca/Oxford COVID-19 vaccine restarted in the United Kingdom after receiving authorization from the MHRA (Medicines Health Regulatory Authority) that the candidate was safe to move forward. The trial sponsors, AstraZeneca and Oxford University stated that they could not divulge any additional medical information.
READ MORE: AstraZeneca Rekindles the Hope of Vaccine After A Voluntary Halt in Late-Stage Trial
On 8 September 2020, AstraZeneca (NASDAQ:AZN) stated that it had voluntarily halted the Phase 3 clinical trial of its COVID-19 vaccine developed by the Oxford University after a woman participant developed some adverse reaction.
Amid the discussions of the impact of AstraZeneca pausing its COVID-19 vaccine development program, the chief executive of the Company, Pascal Soriot stated that in spite of Phase 3 clinical trial being paused, the vaccine could still be accessible by the end of the year.
On 14 September 2020, AZN share price last traded at US$54.02, up by 0.54% from its previous close. The market capitalization of AZN stood at ~US$141.78 billion.
ALSO READ: It's all about the Vaccine Game: Gears that can put ASX 200 into a transition
Pfizer Targets Regulatory Filing of its COVID-19 Vaccine as soon as October in the US
Pfizer Inc (NASDAQ:PFE) and BioNTech SE (NASDAQ:BNTX) are developing an mRNA-based COVID-19 vaccine. Pfizer/BioNTech’s COVID-19 vaccine is under late-stage clinical trial.
On 12 September 2020, Pfizer Inc and BioNTech disclosed that they had submitted a revised protocol for expanding the enrollment of Phase 3 pivotal COVID-19 vaccine trial to up to 44k volunteers to the US Food and Drug Administration (FDA). This way, the companies would be able to enroll new populations.
The companies disclosed that based on current infection rates, they continue to anticipate that a piece of conclusive information on efficacy is likely by the October end.
From the expanded late-stage study, the COVID-19 vaccine candidate of Pfizer and BioNTech may have data in the upcoming weeks. The two partners stated last week that they are on track for a regulatory filing in the United States as soon as October 2020.
On 14 September 2020, PFE share price last quoted at US$37.01, up by 2.61% from its last close. The market capitalization of PFE was noted at almost US$205.66 billion.

.jpg)

