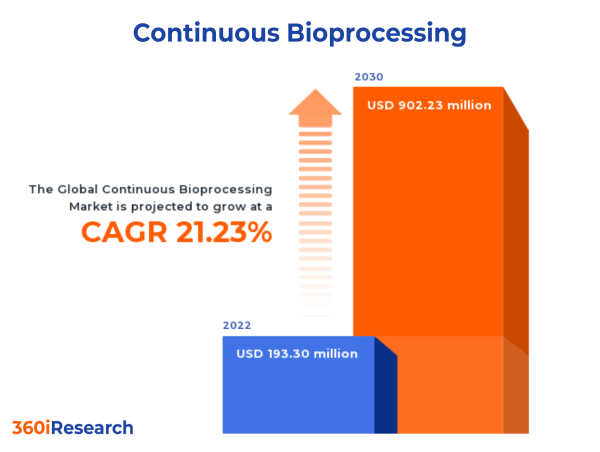
The Global Continuous Bioprocessing Market to grow from USD 193.30 million in 2022 to USD 902.23 million by 2030, at a CAGR of 21.23%.
PUNE, MAHARASHTRA, INDIA , December 7, 2023 /EINPresswire.com/ -- The "Continuous Bioprocessing Market by Product (Bioreactors, Cell Culture Media & Reagent, Centrifuges), Process (Downstream Bioprocess, Upstream Bioprocess), Application, Scale of Operation, End-User - Global Forecast 2023-2030" report has been added to 360iResearch.com's offering.The Global Continuous Bioprocessing Market to grow from USD 193.30 million in 2022 to USD 902.23 million by 2030, at a CAGR of 21.23%.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/continuous-bioprocessing?utm_source=einpresswire&utm_medium=referral&utm_campaign=sample
Continuous bioprocessing represents a paradigm shift in the production of biopharmaceutical products, such as vaccines, monoclonal antibodies, or therapeutic proteins. Continuous bioprocessing allows for the uninterrupted flow of materials and products through the production cycle, offering potential improvements in efficiency, quality control, and scalability, unlike traditional batch processing, which processes a fixed volume of products in discrete steps. Pharmaceutical industries are advancing, and government support for the sector is the principal driver of growth for continuous bioprocessing. Increased benefits of over-batch processing & uptake of single-use technology and the continued demand for the production of biopharmaceutical products are spurring the adoption of continuous bioprocessing technologies. In contrast, high equipment costs, operational difficulties, and process change concerns pose a challenge to adopting a continuous bioprocess. In addition, regulatory considerations must be managed, as continuous bioprocessing may necessitate new validation strategies to comply with the stringent standards set by health authorities. However, several market vendors invest in research and development to address many operational challenges in the continuous bioprocessing technologies and further chart to expand the market. Moreover, the adoption of perfusion is associated with single-use equipment, and the development of a continuous bioprocessing approach to stromal cell manufacturing is expected to enhance overall market growth in the coming years significantly.
End-User: Significant role of continuous bioprocessing in CDMO as they prioritize flexibility and service offering enhancements for their clients
Academic and research institutes often focus on fundamental science and proof-of-concept studies in continuous bioprocessing. They are crucial in advancing technology by conducting innovative research, often collaborating with industry partners. Contract development & manufacturing organizations offer outsourced development and manufacturing services to pharmaceutical and biotech companies. They are an integral part of the pharmaceutical supply chain and increasingly incorporate continuous bioprocessing to differentiate their offerings and improve cost efficiencies. Pharmaceutical and biotechnology companies are the primary end-users driving the commercial implementation of continuous bioprocessing. In comparison, academic and research institutes contribute to the continuous bioprocessing through publications, patents, and training of skilled professionals. In contrast to research institutes, CDMOs invest in scalable and adaptable technologies that can cater to multiple products and processes, optimizing time-to-market for their clients. Pharmaceutical & biotechnology companies carry out both in-house product development and commercial production, unlike academic institutes or CDMOs.
Product: Growing preference for bioreactors in large-scale production of recombinant proteins
Bioreactors are vessels in which biological reactions are carried out, especially for culturing organisms such as bacteria, yeast, and mammalian cells. They are crucial for scaling up bioprocessing. Cell culture media and reagents provide the essential nutrients required for cell growth and product expression in bioprocessing. Centrifuges are utilized to separate components of a mixture based on their density. In bioprocessing, they are essential for cell clarification and product recovery. Chromatography systems are employed for the purification of biological products. Continuous chromatography technologies, including simulated moving bed (SMB) systems, offer higher productivity and better utilization of chromatography media, reducing the cost of consumables. Filtration systems are imperative for sterilizing and clarifying process fluids, warranting the removal of impurities and contaminants. Incubators and shakers are vital for optimizing growth conditions for cell cultures, providing control over temperature, humidity, and agitation. Mixing systems ensure homogeneity in bioreactors and holding tanks, influencing cell growth, nutrient distribution, and product formation consistency. In comparison, continuous bioreactors operate without interruption, ensuring consistent product quality and potentially lower manufacturing costs, and continuous centrifuges allow for the uninterrupted processing of bioproducts, enhancing efficiency and consistency.
Scale of Operation: Growing application of continuous bioprocessing in the clinical operations
Clinical operations are integral to continuous bioprocessing, pivotally influencing the efficacy and quality of the developed biologics. It encapsulates a broad range of activities, from early-stage clinical trials to procedures adhering to regulatory guidelines for the safety and efficacy of medicinal products. Commercial operations in continuous bioprocessing revolve around the practicality of implementing the processes on a large scale for commercialization and sale. It’s about scaling up production and ensuring consistent quality, cost-effectiveness, and regulatory compliance. While continuous bioprocessing presents a promising approach to streamline biopharmaceutical production, its implementation differs widely across the scale, clinical, and commercial operations.
Process: Rising usage of upstream processes owing to progress in terms of efficiency and capacity
Upstream processing, constituting the initial steps of bioproduction, involves developing and maintaining a suitable environment for the growth and multiplication of microbial cells, plant, or mammalian cells. This phase often includes media preparation, cell culture, cell preservation, and primary recovery. Downstream bioprocessing, which follows the upstream step, involves purifying these biological products, and preparing them for their final use or further formulation. Due to inherent complexity and diversity of biological products, downstream processes typically entail complex separation and purification techniques, including centrifugation, filtration, chromatography and more. Both upstream and downstream bioprocessing are critical for both productivity and quality of end products. However, they differ significantly in their chief objectives, complexities, and challenges. Upstream focuses on fostering an optimal environment for cell culture and propagation, while downstream is centered around the purification and refinement of the resultant product.
Application: Extensive utilization of continuous bioprocessing in the vaccine industry to improve public health
Continuous bioprocessing in cell and gene therapies involves using cutting-edge techniques to manufacture personalized treatments. This sector benefits from reduced batch-to-batch variability, enhanced scalability, and improved product consistency. Continuous processes for manufacturing monoclonal antibodies increase productivity and cost-effectiveness due to reduced labor and facility footprint and the potential for real-time quality monitoring. The vaccine industry is experiencing a growing interest in continuous bioprocessing, given the need for rapid development and deployment during pandemics and the production of seasonal vaccines, such as those for influenza. Continuous bioprocessing can substantially shorten development times and improve responsiveness to public health needs. In comparison, continuous processing offers opportunities for enhancing process intensification, stability, and throughput for vaccines.
Regional Insights:
In the American region, the adoption of continuous bioprocessing is driven by a strong focus on innovation in biomanufacturing, significant investments in the biotech sector, and a regulatory environment encouraging advanced production techniques. With major pharmaceutical companies and biotech startups striving to achieve more efficient, cost-effective, and scalable manufacturing processes, continuous bioprocessing offers a competitive advantage. Additionally, the presence of a robust technological infrastructure and highly skilled workforce has facilitated the transition from traditional batch processes to continuous approaches in the Americas. Furthermore, in EMEA, high operational costs and stringent regulatory standards have pushed companies to adopt continuous bioprocessing to reduce costs and enhance the quality of biologics. The European pharmaceutical landscape is known for its strong emphasis on innovation and sustainability, which is further reflected in its rapidly growing biosimilar market, where continuous bioprocessing delivers substantial benefits in terms of production agility and speed to market. Additionally, adopting such processes is nascent in the Middle East and Africa. In this region, the continuous bioprocessing is expected to grow by the increasing need for biologics and efforts to localize pharmaceutical production capabilities. The APAC region has seen a surge in adopting continuous bioprocessing due to its rapidly expanding biopharmaceutical sector and the need to compete globally. A growing patient population needs high-quality biologics, increasing investments from players in biotechnology, and supportive government initiatives aiming to boost biomanufacturing capabilities. Several countries have demonstrated significant interest in incorporating continuous processes to accelerate drug development and production timelines, reduce manufacturing footprints, and enable cost savings, all while complying with international quality standards.
FPNV Positioning Matrix:
The FPNV Positioning Matrix is essential for assessing the Continuous Bioprocessing Market. It provides a comprehensive evaluation of vendors by examining key metrics within Business Strategy and Product Satisfaction, allowing users to make informed decisions based on their specific needs. This advanced analysis then organizes these vendors into four distinct quadrants, which represent varying levels of success: Forefront (F), Pathfinder (P), Niche (N), or Vital(V).
Market Share Analysis:
The Market Share Analysis offers an insightful look at the current state of vendors in the Continuous Bioprocessing Market. By comparing vendor contributions to overall revenue, customer base, and other key metrics, we can give companies a greater understanding of their performance and what they are up against when competing for market share. The analysis also sheds light on just how competitive any given sector is about accumulation, fragmentation dominance, and amalgamation traits over the base year period studied.
Key Company Profiles:
The report delves into recent significant developments in the Continuous Bioprocessing Market, highlighting leading vendors and their innovative profiles. These include 3D Biotek LLC, 3M Company, Adolf Kühner AG, bbi-biotech GmbH, Belach Bioteknik AB, Bio-Rad Laboratories, Inc., Bionet Servicios Técnicos S.L., Colder Products Company, Danaher Corporation, Esco Aster Pte Ltd., Esco VacciXcell, FiberCell Systems Inc., Fujifilm Holdings Corporation, GE HealthCare Technologies Inc., GEA Group, Getinge AB, Infors AG, Merck KGaA, Repligen Corporation, Sartorius AG, simAbs NV, Suzhou Transcenta Therapeutics Co., Ltd., Thermo Fisher Scientific Inc., Watson-Marlow Fluid Technology Group, and WuXi Biologics Co., Ltd..
Inquire Before Buying @ https://www.360iresearch.com/library/intelligence/continuous-bioprocessing?utm_source=einpresswire&utm_medium=referral&utm_campaign=inquire
Market Segmentation & Coverage:
This research report categorizes the Continuous Bioprocessing Market in order to forecast the revenues and analyze trends in each of following sub-markets:
Based on Product, market is studied across Bioreactors, Cell Culture Media & Reagent, Centrifuges, Chromatography System, Filtration Systems, and Incubators & Shakers. The Bioreactors is projected to witness significant market share during forecast period.
Based on Process, market is studied across Downstream Bioprocess and Upstream Bioprocess. The Downstream Bioprocess is projected to witness significant market share during forecast period.
Based on Application, market is studied across Cell & Gene Therapy, Monoclonal Antibodies, and Vaccines. The Monoclonal Antibodies is projected to witness significant market share during forecast period.
Based on Scale of Operation, market is studied across Clinical Operations and Commercial Operations. The Clinical Operations is projected to witness significant market share during forecast period.
Based on End-User, market is studied across Academic & Research Institutes, Contract Development & Manufacturing Organizations, and Pharmaceutical & Biotechnology Companies. The Pharmaceutical & Biotechnology Companies is projected to witness significant market share during forecast period.
Based on Region, market is studied across Americas, Asia-Pacific, and Europe, Middle East & Africa. The Americas is further studied across Argentina, Brazil, Canada, Mexico, and United States. The United States is further studied across California, Florida, Illinois, New York, Ohio, Pennsylvania, and Texas. The Asia-Pacific is further studied across Australia, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. The Europe, Middle East & Africa is further studied across Denmark, Egypt, Finland, France, Germany, Israel, Italy, Netherlands, Nigeria, Norway, Poland, Qatar, Russia, Saudi Arabia, South Africa, Spain, Sweden, Switzerland, Turkey, United Arab Emirates, and United Kingdom. The Americas commanded largest market share of 36.92% in 2022, followed by Europe, Middle East & Africa.
Key Topics Covered:
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
6. Continuous Bioprocessing Market, by Product
7. Continuous Bioprocessing Market, by Process
8. Continuous Bioprocessing Market, by Application
9. Continuous Bioprocessing Market, by Scale of Operation
10. Continuous Bioprocessing Market, by End-User
11. Americas Continuous Bioprocessing Market
12. Asia-Pacific Continuous Bioprocessing Market
13. Europe, Middle East & Africa Continuous Bioprocessing Market
14. Competitive Landscape
15. Competitive Portfolio
16. Appendix
The report provides insights on the following pointers:
1. Market Penetration: Provides comprehensive information on the market offered by the key players
2. Market Development: Provides in-depth information about lucrative emerging markets and analyzes penetration across mature segments of the markets
3. Market Diversification: Provides detailed information about new product launches, untapped geographies, recent developments, and investments
4. Competitive Assessment & Intelligence: Provides an exhaustive assessment of market shares, strategies, products, certification, regulatory approvals, patent landscape, and manufacturing capabilities of the leading players
5. Product Development & Innovation: Provides intelligent insights on future technologies, R&D activities, and breakthrough product developments
The report answers questions such as:
1. What is the market size and forecast of the Continuous Bioprocessing Market?
2. Which are the products/segments/applications/areas to invest in over the forecast period in the Continuous Bioprocessing Market?
3. What is the competitive strategic window for opportunities in the Continuous Bioprocessing Market?
4. What are the technology trends and regulatory frameworks in the Continuous Bioprocessing Market?
5. What is the market share of the leading vendors in the Continuous Bioprocessing Market?
6. What modes and strategic moves are considered suitable for entering the Continuous Bioprocessing Market?
Read More @ https://www.360iresearch.com/library/intelligence/continuous-bioprocessing?utm_source=einpresswire&utm_medium=referral&utm_campaign=analyst
Mr. Ketan Rohom
360iResearch
+ +1 530-264-8485
[email protected]