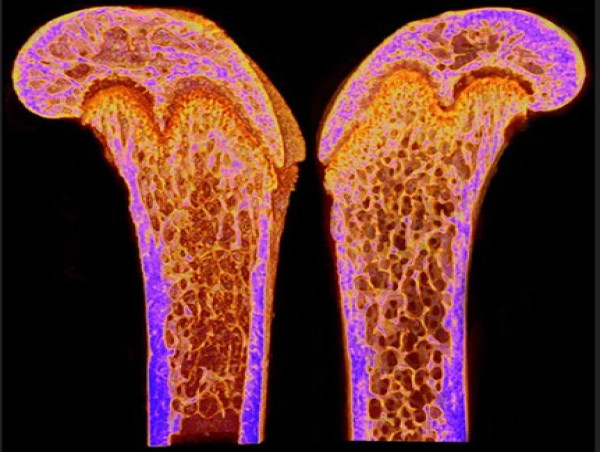
Premature aging weakens bone integrity by impairing osteocyte networks, with male mice showing greater structural and cellular decline
CHINA, June 17, 2025 /EINPresswire.com/ -- A study from ETH Zurich shows that premature aging disrupts bone structure and osteocyte networks in an age- and sex-dependent manner. Using the PolgA mouse model, researchers found significant declines in bone density, osteocyte morphology, and lacuno-canalicular connectivity by 40 weeks of age. Male mice exhibited more severe deterioration. Advanced imaging and computational analysis confirmed the damage. The findings position PolgA mice as a valuable model for studying cellular mechanisms underlying age-related bone loss.
Age-related bone loss is a pressing health challenge for aging populations worldwide. As people age, their bones gradually lose density and become frail, increasing the risk of fractures and reducing their quality of life. Although osteoporosis is typically associated with postmenopausal women, recent studies show that men are also significantly affected. Compounding the issue, the precise cellular mechanisms that drive bone degeneration with age remain poorly understood. Increasingly, research attention has turned to osteocytes—specialized bone cells embedded within the bone matrix—as crucial players in skeletal aging.
Osteocytes form a vast cellular communication network within the bone, known as the lacuno-canalicular network (LCN), which helps regulate bone remodeling. These cells respond to mechanical signals and secrete biochemical cues that influence bone formation and resorption. However, with aging, this network becomes fragmented, and osteocyte function declines. A fundamental question in aging research is how age, and potentially sex, alter the morphology and connectivity of the LCN in ways that impair bone health. Understanding this could help develop targeted treatments for osteoporosis.
Now, scientists at ETH Zurich, led by Professor Ralph Müller, have investigated this question using the premature aging mouse model, PolgD257A/D257A (PolgA). The study was published in the journal Bone Research on May 25, 2025. The researchers used advanced imaging techniques and computational analysis to reveal age- and sex-specific degeneration of bone and the LCN as early as 40 weeks of age, a time point comparable to advanced aging in natural mouse models and elderly humans. The degenerative changes were more prominent in male mice.
“Our study shows that PolgA mice experience accelerated skeletal aging with clear structural impairments and reduced osteocyte connectivity,” said Prof. Müller. “This model offers a powerful system for dissecting the cellular mechanisms of age-related bone loss.”
The researchers first evaluated general musculoskeletal health markers, such as body weight, grip strength, gait, and frailty index, at multiple time points up to 40 weeks. Compared to their wild-type counterparts, PolgA mice displayed a steep decline in physical performance and increased frailty, with males exhibiting more pronounced changes. Micro-CT scans of the femur (thigh bone) showed that both cortical (compact) and trabecular (spongy, porous) bone tissue deteriorated significantly in aged PolgA mice. Cortical thickness and bone area declined. Trabecular bone volume was also reduced significantly. These changes mirrored patterns seen in naturally aged mice and elderly humans, strengthening the case for the PolgA model as a proxy for skeletal aging.
Next, the researchers used confocal microscopy and a custom in silico analysis pipeline to analyze the key features of osteocytes and their dendrites—tree-like branches extending from osteocytes. In aged PolgA mice, both males and females exhibited substantial loss in dendrite number, length, and area, along with reduced osteocyte density. Males showed a more severe degeneration, consistent with their more pronounced bone loss.
The researchers also examined the LCN using fluorescein isothiocyanate (FITC) staining on fresh frozen femur sections, which allowed visualization of the canaliculi and lacunae—channels and cavities inside bones. The researchers found reduced lacunar density, shorter canaliculi, and overall lower network connectivity in aged PolgA mice. Using connectomics tools, they quantified changes in network nodes and distances between osteocytes, showing a clear disruption in osteocyte communication.
“The LCN in aged PolgA mice became sparse and fragmented,” said Prof. Müller. “This not only reflects impaired osteocyte function but could also explain the loss of bone mass and strength.”
In addition to the effects of age on osteocytes and bone health, the study highlights some sex-specific differences. Aged male PolgA mice showed more severe degeneration in several parameters compared to females, possibly due to the protective effects of estrogen. The authors suggest that sex hormones may mitigate some aspects of mitochondrial dysfunction in aging cells, a hypothesis that warrants further investigation.
This study offers a robust model of how aging impairs the very cells that hold our bones together. Moreover, the implications of these findings extend beyond the PolgA model. By validating that this mouse strain recapitulates key aspects of natural aging, researchers now have a tractable system to test interventions aimed at preserving bone health.
“Our work underscores the importance of osteocytes in maintaining bone health as we age,” concludes Prof. Müller. “By understanding how their structure and connectivity degrade, we move closer to identifying new strategies to prevent or reverse bone fragility.”
***
Reference
Titles of original papers: Age- and sex-specific deterioration on bone and osteocyte lacuno-canalicular network in a mouse model of premature aging
Journal: Bone Research
DOI: 10.1038/s41413-025-00428-x
Yini Bao
Bone Research Editorial Office
+86 2885546461
[email protected]
Visit us on social media:
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()