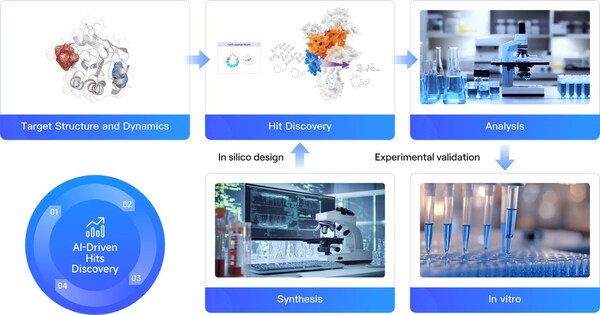
BEIJING, Jan. 9, 2025 /PRNewswire/ -- In a recent achievement, DP Technology's hit discovery platform RiDYMO® has successfully designed a cyclic peptide targeting β-catenin, a historically "undruggable" protein, in just two months. This innovative AI-driven approach combines advanced algorithms, physics simulations, and high-throughput experimentation to rapidly develop potential drug candidates for challenging targets. The platform's success with β-catenin, a key player in cancer development, demonstrates its potential to revolutionize drug discovery for previously intractable targets.
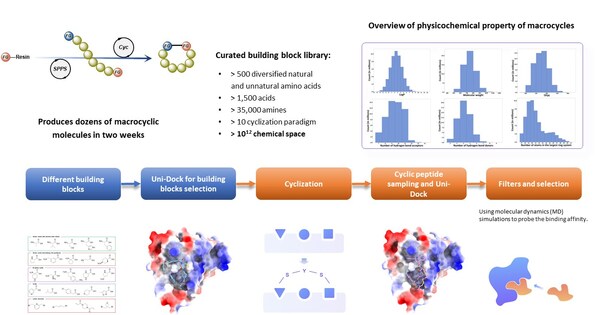
The Wnt/β-catenin pathway plays a crucial role in various biological functions and is implicated in multiple cancers[1-3]. Despite its importance as a therapeutic target[4-7], β-catenin has long been considered "undruggable" due to its flat protein surface[8-10]. The RiDYMO® platform overcomes this challenge by leveraging a vast library of over 10^12 cyclic peptides and macrocyclic molecules, incorporating both natural and non-natural amino acids.
Using the RiDYMO® platform, researchers rapidly designed and screened cyclic peptide molecules for β-catenin binding. The process involved:
- Optimizing the β-catenin protein structure to improve the binding interface.
- High-throughput screening and design of cyclic peptides using the platform's extensive library.
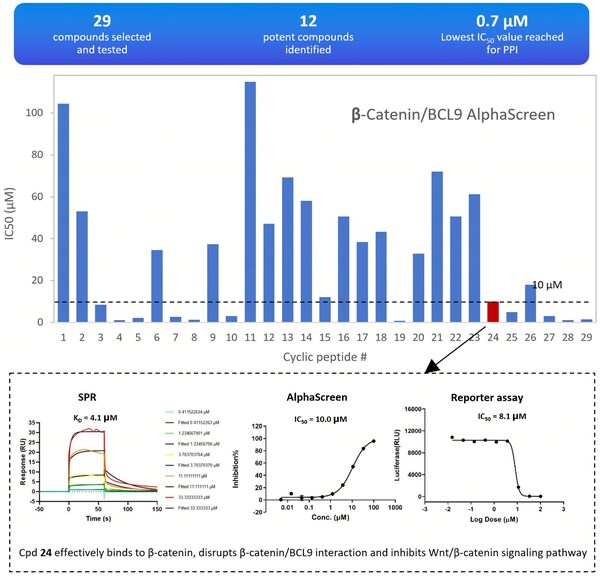
- Synthesis and testing of 29 candidate molecules.
The results were impressive, with 12 compounds showing strong inhibition of protein-protein interactions (IC50≤10 μM), a success rate of over 40%. One standout compound, designated as compound 24, effectively bound to β-catenin, disrupted its interaction with BCL9, and inhibited the Wnt/β-catenin signaling pathway.
Dr. Dongdong Wang, Co-President of DP Technology's Drug Discovery Unit, emphasized the platform's ability to tackle undruggable targets and rapidly design high-quality compounds. The RiDYMO® platform's success with β-catenin demonstrates its potential to accelerate the development of orally available cyclic peptide drugs, potentially transforming the pharmaceutical industry's approach to challenging targets.
This breakthrough in cyclic peptide design for β-catenin showcases the power of AI-driven drug discovery platforms in addressing previously intractable targets, potentially opening new avenues for cancer treatment and other therapeutic areas.
About the RiDYMO® Hit Discovery and Optimization Platform
RiDYMO® is a state-of-the-art hit discovery and optimization platform developed by DP Technology, leveraging the principles of AI for Science. It employs the proprietary Hermite® computational drug design software to elucidate the dynamics of "undruggable" targets and explores a broader chemical space encompassing small molecules, macrocycles, and cyclic peptides. By integrating advanced artificial intelligence, physics-based algorithms, and high-throughput experimentation, the platform excels in designing oral macrocyclic compounds and rapidly delivers innovative drug candidates.
As one of its core algorithms, Reinforced Dynamics (RiD)[4] has a significant advantage in the sampling efficiency of molecular dynamics simulation. By fully leveraging the high-dimensional representation capabilities of neural networks, RiD can efficiently capture dynamic conformational changes in complicated biomolecular systems. Previously, the core RiD algorithm of the platform was published in Nature Computational Science [11]. The study demonstrated that RiD could achieve a more comprehensive free energy surface within 1.86 μs, compared to 100 μs required by traditional MD methods, representing nearly a hundredfold increase in efficiency.
RiDYMO® has been successfully employed in various drug discovery programs, including the development of small molecule compounds for crucial targets such as c-Myc and GPX4, as well as other modalities such as cyclic peptides and ADCs.
For more information, please visit our website.
(https://www.dp.tech/en/services/medicine).
About Hermite
Hermite® is a next-generation computational drug design platform developed by DP Technology, powered by AI for Science, to provide a comprehensive solution for drug design. Hermite® integrates industry-leading tools such as the Free Energy Perturbation module ("Uni-FEP"), and the ultra-high-throughput virtual screening tool ("Uni-VSW"). The platform supports key stages of drug discovery from protein structure prediction and target validation to hit discovery and lead optimization.
In addition, the platform offers an interactive, web-based molecular visualization experience, with detailed management of projects, teams, and data. It features full compliance certification, multi-tier security measures. Users have the flexibility to choose between cloud-based or private deployment options.
Hermite® is rapidly gaining traction in the pharmaceutical industry, with over 60% of leading companies in China utilizing the platform across more than 50 drug pipelines. This growing trust is reflected in the impressive number of calculations performed—over 200,000 Uni-FEP calculations to date—demonstrating Hermite®'s reliability and effectiveness as a powerful tool for drug discovery and development.
For more information, please visit: https://www.dp.tech/en/product/hermite
References:
- Salik B, et al. Targeting RSPO3-LGR4 signaling for leukemia stem cell eradication in acute myeloid leukemia. Cancer Cell. 2020;38(2):263–78.e6.
- Liu J, et al. Wnt/β-catenin signal ling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022 Jan 3;7(1):3.
- Choi BR, Cave C, Na CH, Sockanathan S. GDE2-dependent activation of canonical wnt signaling in neurons regulates oligodendrocyte maturation. Cell Rep. 2020;31(5):107540.
- Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J. Natl. Cancer Inst. 2017;109(8).
- Belenguer G, et al. RNF43/ZNRF3 loss predisposes to hepatocellular-carcinoma by impair ing liver regeneration and altering the liver lipid metabolic ground-state. Nat. Commun. 2022;13(1):334.
- Cao L, et al. Helicobacter pylori-induced RASAL2 Through Activation of Nuclear Factor-κB Promotes Gastric Tumorigenesis via β-catenin Signaling Axis. Gastroenterology. 2022 May;162(6):1716-1731.e17.
- Hashemi M, et al., Biological functions and molecular interactions of Wnt/β-catenin in breast cancer: Revisiting signaling networks. Int. J. Biol. Macromol. 2023 Mar 31:232:123377.
- Hwang et al., Direct Targeting of b-Catenin by a Small Molecule Stimulates Proteasomal Degradation and suppresses Oncogenic Wnt/β-Catenin Signaling. 2016, Cell Rep. 16, 28–36.
- Tanton et al., A novel β-catenin/BCL9 complex inhibitor blocks oncogenic Wnt signaling and disrupts cholesterol homeostasis in colorectal cancer. 2022, Sci. Adv. 8, eabm3108.
- Scuoppo C. et al., "ST316, a Clinical Peptide Antagonist of β-catenin, Induces Anti-Tumor Immune Responses by Multiple Mechanisms of Action." AACR 2024.
- Wang D, et al. Efficient sampling of high-dimensional free energy landscapes using adaptive reinforced dynamics. 2022, Nat. Comp. Sci. 2, 20.