Noxopharm Limited (ASX:NOX) has recently provided an update related to the research progress on its key drug candidate Veyonda® which is presently undergoing clinical trials in late-stage, progressive and metastatic, castration-resistant prostate cancer (mCRPC) patients with limited survival chances.
The interim data of Noxopharm’s Veyonda®/DARRT-1 treatment regimen suggested significant anti-cancer effects in late-stage prostate cancer patients. The data (on half of the study patients after 6 months review) will be presented at the Annual Scientific Meeting of the Clinical Oncology Society of Australia (COSA), scheduled on 12-14 November 2019.
Veyonda® is being developed for safe and more effective radiotherapy for prostate cancer with the ultimate goal to establish Veyonda® as a vital adjunct to radiotherapy at multiple stages of the disease continuum; the DARRT (Direct and Abscopal Response to Radiotherapy) program being the central element of that goal.
Noxopharm’s novel immuno-oncology DARRT-1 treatment regimen aims to achieve a significantly higher effect with a whole-of-body anticancer outcome, combining individual effects of low-dose radiotherapy and Veyonda® on patients’ immune system.
DARRT-1 treatment regimen
In the DARRT-1 Program, the ability of Veyonda® to achieve an anti-cancer tumour response in the body during and after radiotherapy is being delivered to just one or two individual tumours. The aim is to achieve greater pain relief, longer survival in patients and better quality of life, where palliative care is the current standard of care.
The study includes a 5-day course of radiotherapy with Veyonda® administered daily for up to 2 weeks and is divided into two stages-
- Dose escalation arm- 14 men are administered with 400mg/800mg/1200mg Veyonda® coupled with low dose radiotherapy.
- Dose expansion arm- further 11 men are given 1200mg Veyonda® coupled with low dose
Of these 25 men who were grouped together for this analysis, 22 men were included in the current data review who had radiographically evaluable tumours.
Up till now, six months follow-up is being completed in 11 of these men, while the remaining 11 are expected to complete their follow-up in mid-November 2019 with the data to be revealed in November 2019.
It is to be noted that the clinical data is recorded for 6 months and to determine the long-term durability of the anti-cancer effect, patients’ survival will be observed by Noxopharm at 24 months.
Noxopharm’s interim six months data revealed promising results indicating DARRT treatment regimen to be on the right path to meet these objectives.
DARRT-1 study results
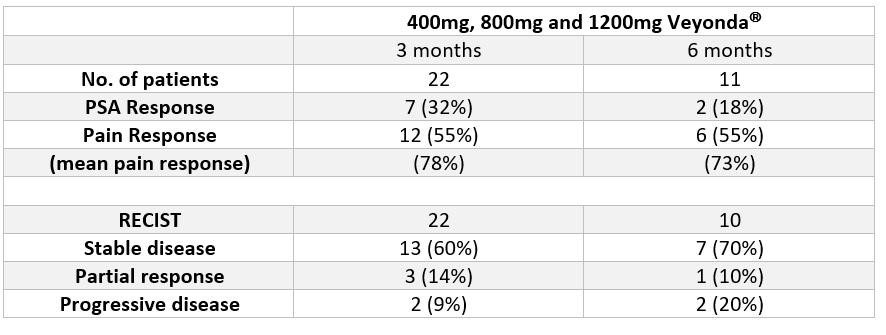
In 8 of 10 patients (80%) evaluated over a 6-months period of observation, the cancer progression was found blocked. In addition, a clinically significant decrease in pain levels was observed in more than half of the patients (55%).
The table below summarises the data from all patients in the study at 3-months & 6-months review points.
Noxopharm expects to release the final statistical study report in early-2020, comprising a comprehensive radiographic evaluation of both irradiated and non-irradiated tumour responses.
DARRT-1 Previous results
Interestingly, DARRT-1 Dose-Escalation study results revealed long-lasting anti-cancer response rate for up to six months (the length of the study) in terms of tumour size, pain and prostate specific antigen (PSA) response in a high proportion (57 per cent) of men with end-stage mCRPC. These patients were dosed with a short, minimally intrusive, well-tolerated regimen of Veyonda® in combination with radiotherapy.
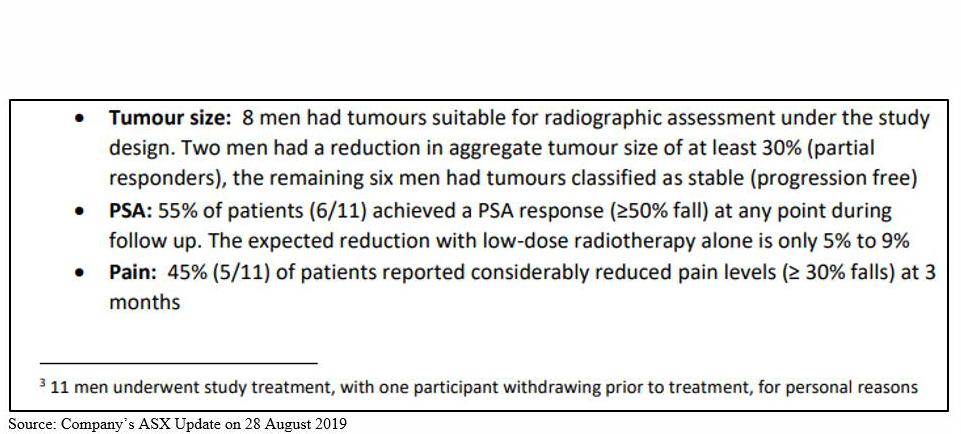
Earlier, Noxopharm announced promising 3-month Results for DARRT-1 Dose Expansion Arm indicating a significant reduction in pain in 45% of men, stable tumour size or overall shrinkage in all radiographically evaluable patients and a PSA response in 55% of men.
Presently, Noxopharm plans to initiate the next stage of the DARRT program in prostate cancer by early 2020, aiming to make the DARRT-2 study an American and European-based study in late-stage prostate cancer.
Noxopharm’s stance for 2020: A promising and strongly determined clinical agenda for progressing the commercialisation of Veyonda®.
Noxopharm Executive Chairman, Dr Graham Kelly is pleased with the evident anti-cancer effect from the DARRT therapy, especially in men who have run out of treatment options, which he believes is an important milestone. With the current focus on establishing DARRT in prostate cancer, Noxopharm foresees this treatment may apply to a wide range of cancers.
NOX is currently trading at $0.375 (AEST 02:55 pm) on 29 October 2019.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. The above article is sponsored but NOT a solicitation or recommendation to buy, sell or hold the stock of the company (or companies) under discussion. We are neither licensed nor qualified to provide investment advice through this platform.