Highlights
- IMU is focused on advancing four platform technologies , Allo CAR T cell Therapy, CF33 Oncolytic Virus, onCARlytics and B Cell Immunotherapy.
- The company is backed by an experienced management team with over 150 years of combined experience in drug development & approvals.
- Clinical trials are underway in blood cancer and diverse solid tumors.
Imugene Limited (ASX: IMU) is an ASX-listed biotechnology company which is focused on developing potent and innovative therapies for cancer patients.
As of 30 September 2023, the company had cash of AU$163 million.
The platform technologies under development by the company are-
- Azer-cel – Allogeneic (Allo) CAR T cell therapy
- VAXINIA – CF33 Oncolytic Virus Therapy
- onCARlytics – OnCARlytics CF33-CD19 OV Therapy
- HER-Vaxx and PD1-Vaxx – B Cell Immunotherapy
Let’s look at the recent developments across these technologies:
Azer-cel CD19 Allo CAR-T cell therapy
This Allo CAR T cell therapy has shown positive clinical activities in B Cell Malignancies. To date, 84 patients have been treated with azer-cell, comprising 61 non-hodgkin lymphoma (NHL) patients and 23 B-cell lymphoblastic leukaemia (B-ALL) patients. The overall response rate (ORR) in NHL patients was 58% and complete response (CR) was 41%. While, in B-ALL patients, ORR was 61% and CR was 61%.
Considering the high overall response rate of 83%, the company says that azer-cel has the potential to be a new standard of care.
The company has set its sight on the Phase 2 trial, and the potential registrational study would commence after the conclusion of the Phase 1B study in the second half of 2024.
The registration study is dependent upon the acceptable CR rate and durability of CR.
CF33 Oncolytic virus
The Phase 1 MAST study witnessed substantial enrolment. The MAST study demonstrated durable responses in a heavily pre-treated population.
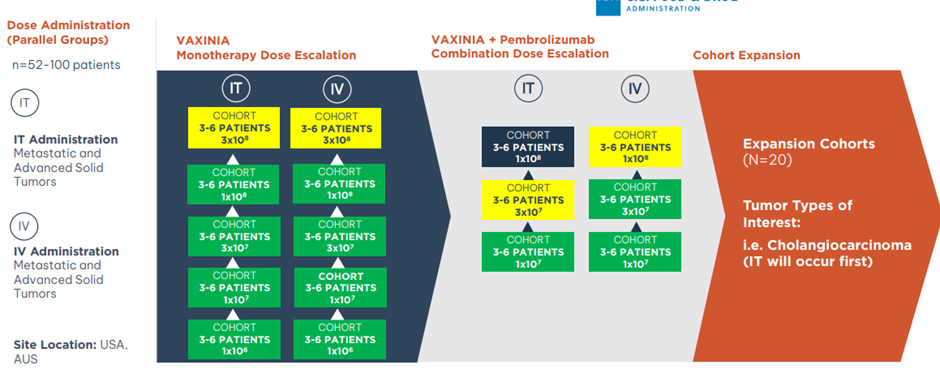
Image source: Company PPT
To date, 32 heavily pretreated patients have been enrolled and 24 were evaluable and have been evaluated. Most patients had control of their disease, and at higher doses, patients showed significant and durable reduction in the tumor burden.
OnCARlytics
OnCARlytics makes solid tumours visible by CD19 targeting therapies. The phase 1 OASIS study is designed to treat only with onCARlytics or in combination with Blinatumomab and either dosed intravenously (IV) or intratumorally (IT) in metastatic cancer advanced patients across multiple solid tumors, For the trial, enrolment had commenced in 2023, with first patient enrolled in October 2023 at City of Hope.
The company intends to conduct Phase 1 study in around 10 sites in the US in 2024. There are many CD19 approved drugs which could become preferred partners to combine with onCARlytics.
B-cell immunotherapies
HER-VAXX HERIZON study demonstrated continued overall survival benefit with an additional 6 months follow up.
The company intends to undertake HER-VAXX phase 2 trial in the USA, Taiwan and Australia on the patients with-
- 2L+
- Advanced or metastatic GJ/GEJ
- Arm 1: HER-2/neu overexpressing at diagnosis
- Progressed on prior trastuzumab, T-DXd or other anti-HER-2 ADC
The trial would focus on a chemotherapy combination in gastric cancer that progressed after Trastuzumab. The primary endpoint will be ORR and safety, while secondary endpoint will be PFS, OS and DoR.
To know how Imugene is advancing on its PD1-Vaxx, read here.
Plans for coming 12-24 months
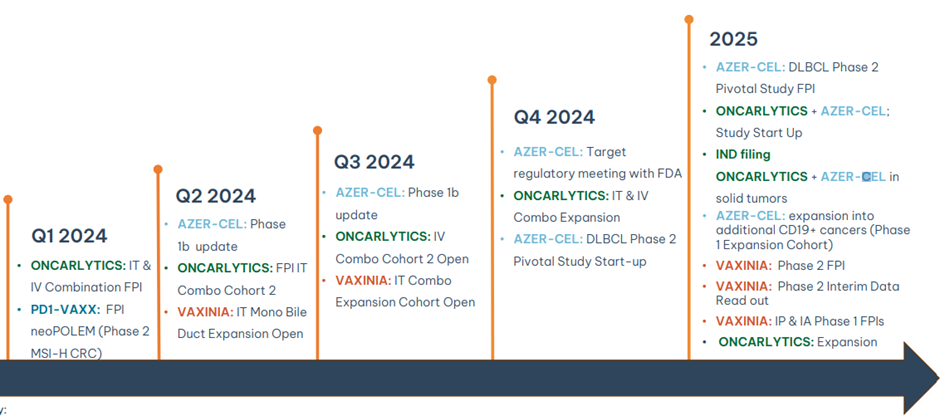
Image source: Company PPT
In essence, Imugene is prioritising opportunities in blood and solid cancers backed by its unique technology platforms.
IMU shares traded at AU$0.125apiece on 10 January 2023.



