Highlights
- Radiopharm has a pipeline of six platforms and well-differentiated molecules.
- The company is studying patients with brain metastasis with its lead product candidate F18 Pivalate.
- The company received positive and encouraging results from Phase II brain metastasis trial with Pivalate.
Radiopharm Theranostics (ASX:RAD) has been making some serious strides to achieve its goal of becoming a recognised leader in innovative radiopharmaceutical therapies for cancer treatment.
Radiopharmaceuticals are radioactive drugs that are safe and can be used as diagnostic or therapeutic tools. Radioactive medication is injected into the blood stream of a patient in a very small amount.
Radiopharmaceuticals are designed to complement surgery and postpone the need for chemotherapy. They are also known to enhance targeted and immune therapies.

© 2022 Kalkine Media®, Data source: Company update
Radiopharm has one of the deepest pipelines in radiopharmaceutical therapies, with six platforms, well-differentiated molecules and potential applications more than 20 clinical development trials in multiple indications.
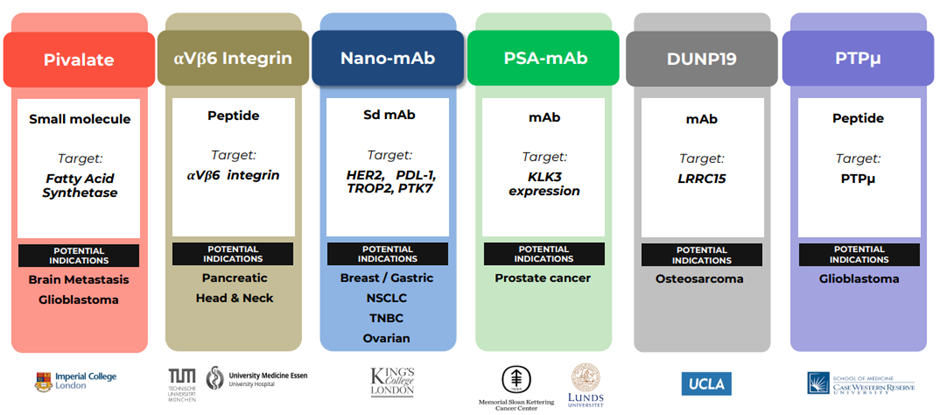
Image source: Company update
Radiopharm’s research targeting brain metastasis
F18 Pivalate is the lead product candidate of Radiopharm for the imaging and treatment of brain metastasis, a condition wherein cancer cells spread from their original site to the brain.
Radiopharm suggests that 20-40% of cancer patients develop metastatic brain cancer during the course of illness. Further, there are several limitations to current imaging techniques such as PET FDG & MRI due to necrotic, inflammatory & high sugar uptake confounding factors.
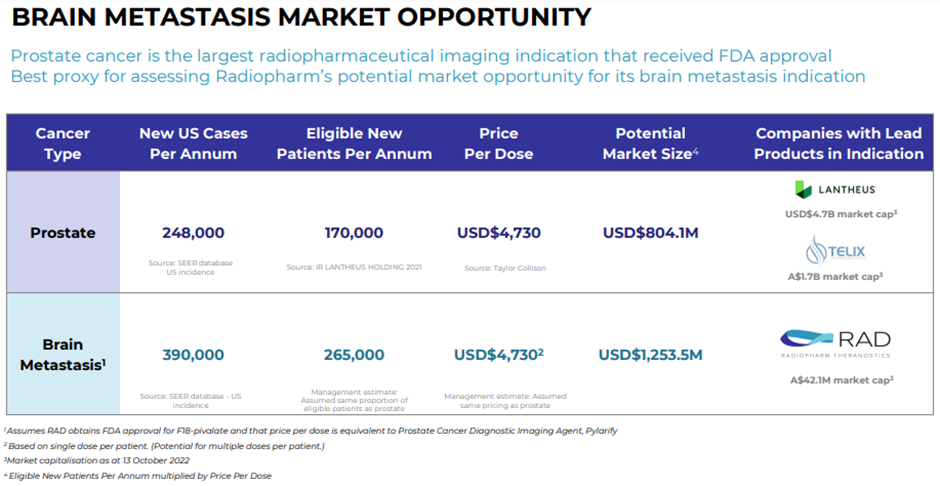
Image source: RAD update
Radiopharm is undertaking study on brain metastasis with its lead product candidate F18 Pivalate.
Pivalate was invented at Imperial College London by Professor Eric Aboagye. It is an 18F-FPIA radiotracer, based on a short chain carbohydrate. It uses the early steps of fatty acid oxidation and is very stable.
Radiopharm holds an exclusive worldwide licence for the Pivalate platform technology from Cancer Research Technology Limited and Imperial College London. The company also has a sponsored research agreement on new analogues with Professor Aboagye.
F-18 Pivalate delivers positive data in Phase II trial
F18 Pivalate selectively targets fatty acid synthetase, which is overexpressed in tumours but not in normal brain cells. It is a novel radiopharmaceutical for identifying, characterising, and monitoring the progress of glioblastoma and brain metastasis, says RAD.
F18-pivalate’s unique mechanism of action & transformational approach is designed to overcome the limitations.
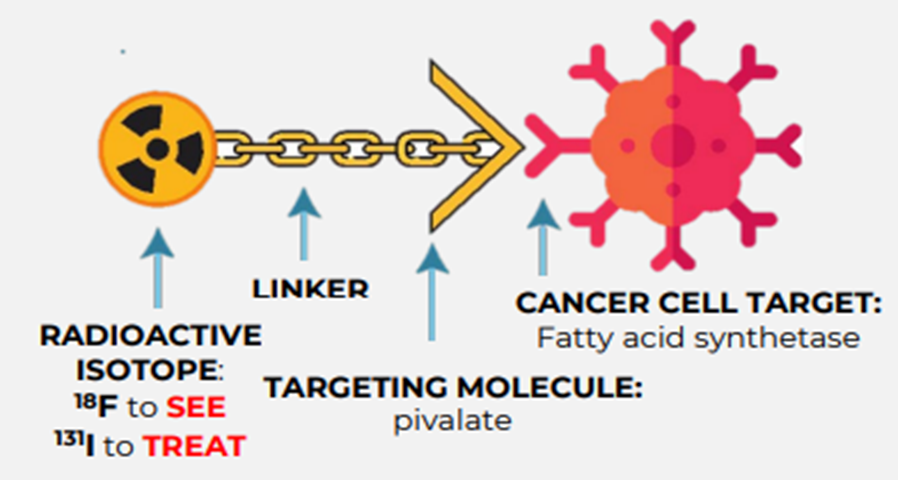
Image source: Company update
In October this year, the company announced that Pivalate delivered positive data in Phase II brain metastasis trial. It showed high uptake regardless of the origin of the primary tumour, implying that it can be used to detect and monitor cerebral metastases.
In addition, patients without previous exposure to external beam radiations showed higher tumour uptake of radiopharmaceutical, whereas previously treated patients showed a reduced trend of uptake.
The road ahead for trial in brain metastasis
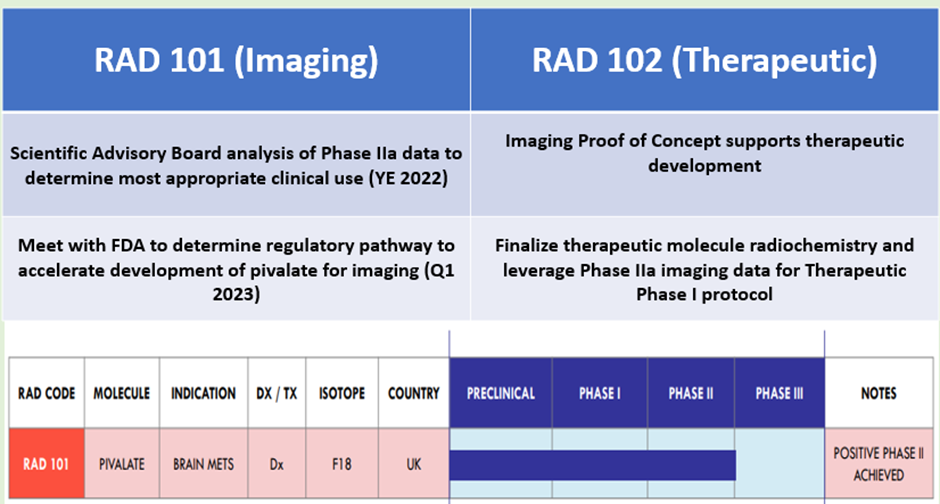
Data source: Company update
Radiopharm shares traded at AU$0.120 on 18 November 2022.