Highlights
- A planned Data Safety Monitoring Board (DSMB) review of Cynata’s diabetic foot ulcer (DFU) clinical trial has been successfully completed.
- The DSMB recommends the company’s DFU clinical trial continue unchanged.
- The DFU clinical trial is aimed at investigating the safety and early efficacy of Cynata’s unique topical Cymerus™ mesenchymal stem cell (MSC) product, CYP-006TK, in patients with DFUs.
Cynata Therapeutics Limited (ASX: CYP), a clinical-stage biotechnology company that specialises in cell therapeutics, has made a significant announcement regarding its DFU clinical trial. An independent DSMB has successfully reviewed progress to date in the company’s DFU clinical trial and recommended continuation.

Image: © 2022 Kalkine Media®
About Cynata’s DFU clinical trial
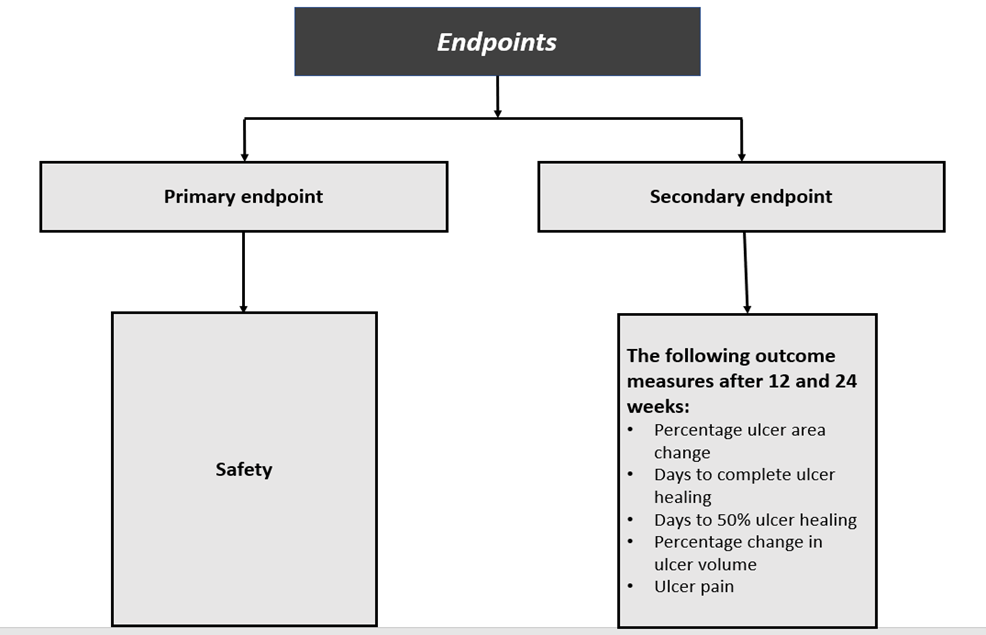
The trial, titled “A Randomised, Controlled, Phase 1 Study to Investigate Safety, Tolerability and Efficacy of CYP-006TK in Adults with Diabetic Foot Ulcers,” will see 30 participants enrolled. These participants will be randomly assigned to receive either CYP-006TK or standard-care treatment.
What is CYP-006TK?
This will be the first clinical trial to use CYP-006TK, which is a polymer-coated silicon dressing (seeded with Cymerus mesenchymal stem cells), which Cynata has licensed from TekCyte Pty Ltd (TekCyte). The investigational treatment period is of 4 weeks, and each patient will be evaluated for 24 weeks.
Date source: Company update
© 2022 Kalkine Media®
What has the DSMB recommended for Cynata’s DFU clinical trial?
After the routine review, the DSMB has made the recommendation that the company’s DFU clinical trial be continued as planned. Importantly, a review by an independent DSMB is in line with Good Clinical Practice.

Date source: Company update
© 2022 Kalkine Media®
The study protocol for the DFU clinical trial includes supervision by a DSMB, along with provision for an interim review, which has been successfully completed now.

Date source: Company update
© 2022 Kalkine Media®
About Cynata
Cynata Therapeutics Limited is an Australian clinical-stage stem cell and regenerative medicine company whose main activities are the development and commercialisation of its proprietary Cymerus™ mesenchymal stem cell (MSC) based therapies.
Cymerus™ is Cynata’s exclusive therapeutic stem cell platform technology for economic manufacture of cell therapy products at commercial scale without the limitation of multiple donors.



