Highlights
- Imugene Limited (ASX:IMU) marked 2022 with breakthrough developments in its clinical studies, progressing to build a deep clinical pipeline
- At present, IMU has five ongoing clinical trials. However, a total of nine clinical trials are expected to be in progress in Phase 1 and Phase 2 studies in next 12 months
- Imugene has three scientific collaborations with two cell therapy companies based in the US - Celularity, Eureka Therapeutics, and one with an ASX-listed company Arovella Therapeutics
- The biotech company ended 2022 with a AU$160million cash reserve, one of the largest cash reserves of any ASX-listed biotech firm as stated by IMU in its news release
Imugene Limited (ASX:IMU), an ASX-listed company engaged in developing cancer immunotherapies, has brought forth the company’s state of affairs along with some observations on 2023 to help its shareholders have a quick recap on the year that was.
As a biotech company, Imugene aims to nail its cornerstone of building a technology that can prevent cancer. With a material breakthrough in its clinical studies, the company believes to have built a deep clinical pipeline unlike other ASX biotech companies.
Here’s a thumbnail sketch of IMU’s clinical progress
Imugene is advancing towards a stronger, well-funded, and balanced portfolio of clinical trials and funds to cross key milestones with its technologies.
The five ongoing clinical trials of the company are
- HERIZON extension (HER-Vaxx, HER2+ gastric cancer)
- nextHERIZON (HER-Vaxx in combination with pembrolizumab (MSD), late-stage HER2+ gastric cancer)
- IMPRINTER (PD1-Vaxx, non-small cell lung cancer) in combination with tencentriq (Roche)
- MAST (Vaxinia, CF33-hNIS oncolytic virus in advanced solid tumors)
- City of Hope IST (CHECKVacc, CF33-hNIS-aPDL1, triple-negative breast cancer (TNBC)) – have seen substantial progress in 2022.
The company initiated the IV dosing arm of the Vaxinia MAST trial in October 2022. Entering 2023, IMU aims to start the combination with MSD’s blockbuster pembrolizumab cohorts in the Vaxinia MAST study. Subjects will be recruited into the higher dose cohorts in CHECKvacc. Also, IND will be filed, and patients will be dosed in the onCARlytics platform. In Phase 2 study for nextHERIZON (relapsed/refractory HER2+ gastric patients), more patients will be dosed.
In the HERIZON study (front line HER2+ gastric), IMU continues to follow patients, some of whom have been on the study for more than two years. Also, dosing is being administered in the PD1-Vaxx Phase 1 trial to reach an optimal biological dose with one Australian patient still in remission from late-stage non-small cell lung cancer after two years of treatment.
In the next year, a total of nine clinical trials are expected to be in progress in Phase 1 and Phase 2 studies.
Overview of business activities
Dr Monil Shah is leading the company’s business development initiatives, profiling all the technologies within the IMU portfolio to parties in the United States, Europe, and Asia.
In a bid to generate data of interest to potential partners, the company is pursuing multiple paths in highly competitive circumstances.
As of now, IMU has three scientific collaborations with two cell therapy companies based in the US - Celularity, Eureka Therapeutics, and Arovella Therapeutics.

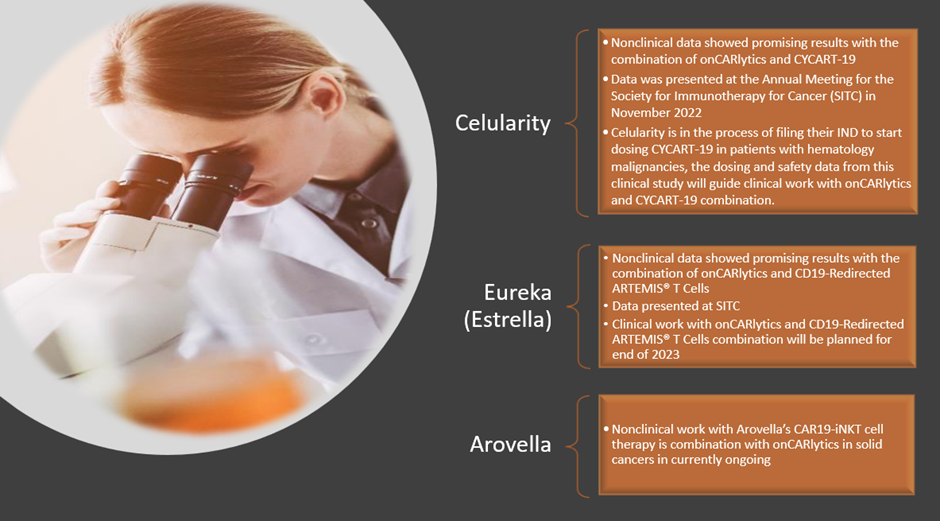
Data source: Imugene update; Image source: © 2023 Krish Capital Pty. Ltd.

Data source: Imugene update; Image source: © 2023 Krish Capital Pty. Ltd.
Working closely with manufacturers for oncolytic viruses
The reliability of drug supply is a critical component of clinical development. Therefore, IMU is aiming to optimise it through oncolytic viruses with the assistance of manufacturers - City of Hope’s Center for Biomedicine and Genetics and ABL; and for the B cell platform: piCHEM, a long-established B cell cancer vaccine manufacturer
In 2022, IMU announced a collaboration with ABL, wherein ABL will produce IMU’s oncolytic viruses for the latter’s platform. This has brought in direct access for IMU to ABL’s top-of-the-line services, including GMP manufacturing of vaccinia viruses and fill-finishing of the drug product, with customisable and flexible development and manufacturing solutions.
Also, in the year 2022, IMU concluded and delivered a batch of HER-Vaxx for use in all planned clinical trials (nextHERIZON and neoHERIZON) in patients having HER-2 positive gastric cancer. In addition, a batch manufactured by piCHEM (Austria) was delivered to a drug depot at Marken (Singapore).
IMU’s message for investors

IMU shares were trading at AU$0.167 each on 09 January 2023.
IMU shares were trading at AU$0.167 each on 09 January 2023.



