Summary
- ASX 300 listed healthcare player Starpharma Holdings Limited receives A$1 million funding from MRFF for its SPL7013 COVID-19 nasal spray.
- The Company intends to utilise the funds to accelerate the development and commercialisation of the antiviral nasal spray.
- SPL7013 has demonstrated significant activity against the novel coronavirus, and Starpharma has confirmed a fast track regulatory pathway for several critical markets.
- Following the announcement, Starpharma share price rose and closed the day’s session at A$1.700, up 4.294% on 3 September 2020.
ASX 300 listed biotech player Starpharma Holdings Limited (ASX:SPL) announced today that it had received funding worth A$1 million from MRFF for its SPL7013 antiviral nasal spray. The news went down well the market participants with SPL share price ending the day’s trade at A$1.700, moving up 4.294%.
Apart from the SPL7013 nasal spray, Starpharma is also engaged in a second program to address COVID-19. Lately, on 1 September 2020, the Company revealed that it had applied its novel DEP® drug delivery technology for developing a long-acting, water-soluble formulation of remdesivir.
About Starpharma Holdings Limited
Healthcare company Starpharma Holdings Limited is engaged in developing therapeutic dendrimer formulations for pharmaceuticals, life sciences, and other applications. The two key development programs of Starpharma include DEP® drug delivery & VivaGel® portfolio.
Starpharma is in the race of combating COVID-19 pandemic with two programs to address COVID-19: SPL7013 nasal spray and DEP® remdesivir.
GOOD READ: 5 Healthcare Share Prices Under Discussion Today - Starpharma, Ramsay, Mesoblast, Ansell, CLINUVEL
Let us delve deep and discuss ASX 300 listed Starpharma’s announcement today-
Starpharma Awarded A$1 million Funding for SPL7013 COVID-19 Nasal Spray
On 3 September 2020, Starpharma disclosed that the Company is awarded A$1 million funding by Medical Research Future Fund (MRFF) Biomedical Translation Bridge (BTB) Program of Government of Australia. Starpharma shall use this funding to accelerate development and commercialisation of its SPL7013 antiviral nasal spray to prevent COVID-19.
The Company was granted A$1 million non-dilutive funding in an extremely competitive COVID-19 specific Medical Research Future Fund funding round. Starpharma’s product was one of the five contenders that were selected for support by an international expert panel for specific MRFF funding for COVID-19.
The funding required projects to be capable of accomplishing significant and instant impact in the global response to the COVID-19 turmoil within one year.
Moreover, Starpharma intends to speed up approval of SPL7013 nasal spray by leveraging its existing regulatory approvals as well as broad clinical & nonclinical information for related products containing SPL7013. The Company will also leverage its current supply chains along with manufacturing connections to accelerate product access.
SPL7013 Nasal Spray:
Patented SPL7013 nasal spray of Starpharma has the potential to prevent both acquisitions as well as transmission of novel coronavirus (SARS-CoV-2). With its broad-spectrum antiviral action, SPL7013 could also play a role for other respiratory viruses and upcoming pandemic preparedness.
Feedback from healthcare providers and clinicians suggests a strong interest in a preventative product for COVID-19 as an additional line of protection, in addition to vaccines and conventional PPE.
The nasal spray would have application for the general population, including those in the frontline of the ongoing crisis, including doctors, nurses and all who are exposed to overcrowded & high-risk environments, such as airlines, public transport, and aged care.
On top of its potent antiviral effects, SPL7013 has several advantages, including being the active component in approved and marketed VivaGel® products sold in Australia, Asia, Canada, Europe, New Zealand, and the United Kingdom. Notably, SPL has already manufactured SPL7013 at commercial scale for its rapid market entry.
Starpharma Reported Potent Antiviral Activity of SPL7013 Against SARS-CoV-2
On 25 August 2020, Starpharma provided a development update on its SPL7013 nasal spray for COVID-19. The Company asserted that additional antiviral testing of SPL7013 had been completed and the data corroborates that SPL7013 inhibits host cells infection caused by novel coronavirus after its application to the cells either before or after virus exposure.
The data confirmed potent antiviral activity against SARS-CoV-2 and provided supporting data on the mechanism of action (MOA), indicating that SPL7013 acts early in the viral replication cycle.
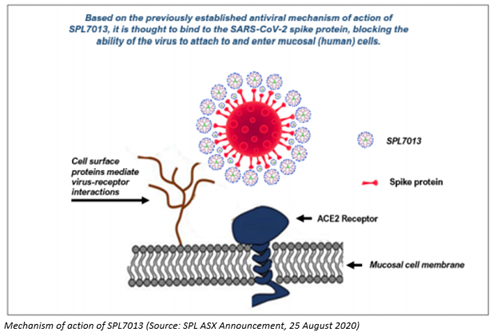
In an assay for detecting virucidal action, SPL7013 also made novel coronavirus inactive & stopped infection when it was mixed with the virus before adding to cells.
The high potency as well as high selectivity index of SPL7013 signify that a final formulated product will have a thousand-fold greater concentration of SPL7013 than the concentration demonstrated to exert an antiviral action on the deadly novel coronavirus.
Starpharma Creates Long-acting Version of Gilead’s Remdesivir
Other than SPL7013 nasal spray Starpharma is also developing slow release soluble form of remdesivir. On 1 September 2020, the Company revealed that it had applied its novel DEP® drug delivery technology to develop a long-acting, water-soluble form of remdesivir to treat COVID-19 patients. The solubility of DEP® remdesivir is 100-fold greater than the standard remdesivir.
TO KNOW MORE DO READ: COVID-19: What Drove ASX 300 listed Starpharma Share Price Today?
Remdesivir is currently under development by Gilead Sciences Inc (NASDAQ:GILD) for treatment of COVID-19 patients. Antiviral drug Remdesivir has EUA (emergency use authorisation) from the US FDA to treat COVID-19 in adults as well as children who are hospitalised with serious symptoms.
RELATED: How Does Gilead’s Antiviral Drug Remdesivir Help to Combat COVID-19?



