Australian clinical-stage drug development company, Noxopharm Limited (ASX:NOX), has provided commentary on clinical data to be released to a cancer conference in November. Noxopharm believes that the data adds to the growing body of evidence that its drug candidate, Veyonda®, is set to become a commonly-used drug in the treatment of prostate cancer.
Veyonda® designed to make radiotherapy safer and more effective. Enormous market opportunity in prostate cancer
One in 7 men can expect to be diagnosed with prostate cancer, and thanks to progress in diagnosis and early treatments, most of these men will not succumb to the cancer. But about 1 in 40 men still will die from the cancer, and this figure hasn’t changed much over the years because there has been virtually no major progress in the treatment of the cancer once it reaches a late stage.
One company that is determined to do something about this is the large Swiss pharma company, Novartis. It paid US$6 billion in 2018 for a technology that it hopes will bring hope for men with late-stage prostate cancer. It isn’t meant to cure them, but it should mean a longer life with a much better quality of life.
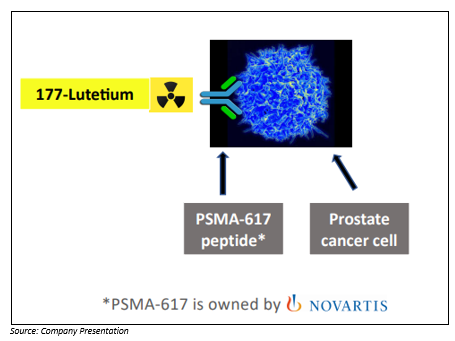
It is a form of radiotherapy known as 177lutetium-PSMA-617 (shortened to 177Lu-PSMA).
177Lu-PSMA is designed to address a major challenge facing the treatment of late-stage prostate cancer. That challenge is that the cancer in most men has spread throughout the body, even to the extent of hundreds of tiny cancers, mostly in the skeleton. There simply are too many cancers to use traditional radiotherapy, so 177Lu-PSMA has been developed as a way of injecting radiation intravenously so that it travels around the body in the blood, finally attaching to the cancer cells wherever they are.
177Lu-PSMA is made up of two parts. One part is a peptide (PSMA-617) that recognizes prostate cancer cells and attaches to them. The second part is the radioactive mineral lutetium-177, linked to the peptide. Once the peptide finds the cancer cell, it enters the cell, taking the radioactive material with it, where it stays for several weeks, emitting radiation that the patient hopes will eventually kill the cancer cell.
177Lu-PSMA is starting to attract a lot of international attention. It already has been used in over 3000 men, and while people are still learning how best to use it, the results so far are good, with a reasonably high proportion of men dying of prostate cancer responding to treatment and showing big reductions in pain and improved health. What isn’t known at the moment, because the technology hasn’t been around long enough, is just how long the benefit will last.
Novartis is trying to answer that question at the moment with a large (750 men) trial in the U.S. and Europe. We should find out the results of that trial towards the end of 2020. If, as we expect, they are successful and get permission to sell the drug in North America and Europe, then a lot of people expect that 177Lu-PSMA will become a standard treatment in late-stage prostate cancer.
Noxopharm believes that Veyonda® will be part of the future of 177Lu-PSMA
No anti-cancer drug works in everyone, and we don’t expect 177Lu-PSMA to be any different. We already know that not every man responds to this drug, and those that do, show a variable effect ranging from moderate to very good. We also anticipate that 177Lu-PSMA therapy will not be cheap, plus it does come with some side-effects, so if a patient is going to commit to 177Lu-PSMA therapy, then he would want to know that his chances of responding are high enough to justify the time (6-8 months) and cost.
Noxopharm believes that Veyonda® will change this equation by boosting the effectiveness of 177Lu-PSMA therapy. If it can achieve this, and do it safely, then both patients and doctors should want to use the combination.
The Company is testing this idea in a Phase 2 clinical study where 56 men with late-stage prostate cancer are getting a combination of Veyonda® and 177Lu-PSMA therapy. This is the first study of its kind where another drug is being used to boost the effectiveness of the Novartis drug. The study is known as the LuPIN Study and is being conducted at St Vincent’s Hospital, one of Sydney’s major teaching hospitals and a pioneer in 177Lu-PSMA therapy.
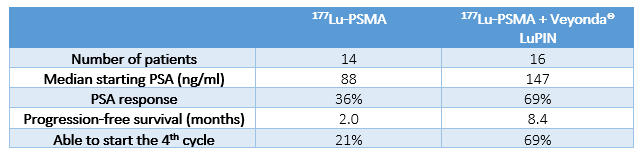
The doctors running the LuPIN Study have released a preliminary report on the first 16 men in the LuPIN Study.
They wanted to see if there was any evidence, even at this early stage, that Veyonda® was providing any additional benefit. To do this, they compared the results from the first 16 men receiving the combination treatment, to the first 14 men the hospital had done with 177Lu-PSMA on its own (no Veyonda®).
The results suggest that, even at this early stage, adding Veyonda® makes a big difference.
The key end-point to focus on is the number of men who were able to reach the 4th cycle of treatment.
177Lu-PSMA therapy involves a series of intravenous injections of the radioactive drug every 6 weeks. Somewhere between 4 and 6 injections (cycles) are given, which means the entire treatment lasts between about 4-6 months. Men only continue to receive injections providing that their cancer doesn’t start re-growing. If it does, then the treatment stops.
That means that the longer a man can remain on treatment, the more likely he is to get a better anti-cancer response.
The results show that in the 14 men receiving 177Lu-PSMA therapy alone, only 3 (21%) managed to make it to the 4th injection of 177Lu-PSMA before their cancer started growing. That is 1 in 5 men.
In the 16 patients who received Veyonda® with each 177Lu-PSMA cycle, 69% managed to make it to the 4th cycle. That is a tripling of the response rate that backs up the Noxopharm belief in the future of Veyonda® as an integral part of the treatment of prostate cancer.
We expect more data to be released over the next 6 months, leading up to the conclusion of the study at the end of 2020.
NOX was trading at $0.380, on October 3, 2019 (AEST 03:05 PM).
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. The above article is sponsored but NOT a solicitation or recommendation to buy, sell or hold the stock of the company (or companies) under discussion. We are neither licensed nor qualified to provide investment advice through this platform.