An Australia-based commercial and clinical-stage biopharmaceutical company, Immuron Limited (ASX: IMC) focuses on treatment of infectious diseases with oral immunoglobulin-based therapies, targeting the human gut immune system and the gut microbiome.
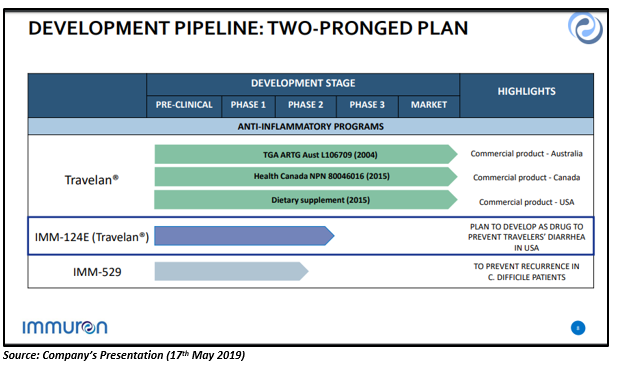
The company has one marketed product, which provides proof of concept for its Oral Immune Technology Platform âTravelan®â which is used for the prevention of travellersâ diarrhoea. Presently, Travelan® is marketed in Australia, USA and Canada.
There are two other clinical assets of the company apart from Travelan®:
- IMM-124E - It is in Phase 2 clinical trials for NASH, ASH and paediatric NAFLD.
- IMM-529 â This asset is in the clinical development for the treatment of Clostridium difficile Infections.
The company released a corporate presentation today on âTargeting Infectious Diseases With Oral Immunotherapiesâ. It was presented by the Chief Executive Officer of the company, Dr Gary Jacob.
Some of the segments discussed in the presentation are highlighted below:
US Department of Defense R&D Collaboration Agreements
The company had collaborated with the following Institutes for the development of a Shigella-specific therapeutic:
- Armed Forces Research Institute of Medical Sciences (AFRIMS) in June 2016
- Naval Medical Research Center (NMRC) in August 2016
- Walter Reed Army Institute of Research (WRAIR) in June 2016
Travelan® Commercial Profile
The global market size of Travelan® is US$630 million in 2019, expected to reach US $890M by 2024 at 7% CAGR. If the Travelan® would be approved as a drug to prevent TD, the sale of the drug is expected to reach more than $100 million in the United States.
IMM-124E Drug Development Plan
The company plans to revamp Travelan® for FDA approval to develop it as a drug to prevent Travelersâ Diarrhea in travellers. The process of the drug development plan is represented below:
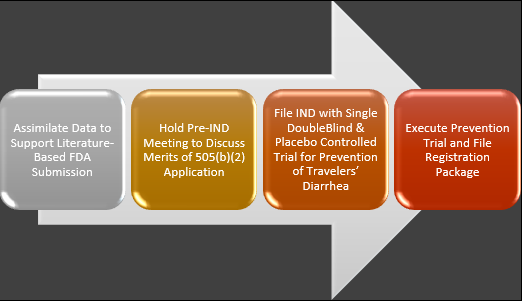
On 11th April 2019, the company announced its plans to pursue clinical development of IMM-124E as a drug to prevent Travelersâ Diarrhea (TD).
Clostridium difficile Market Opportunity
The therapeutic market is expected to reach over $1.7 billion by 2026 from USD $630 million in 2016 at a CAGR of 15 per cent. Also, the C. difficile infection is a leading cause of gastroenteritis-associated mortality in the U.S. Around 44,500 patients died in 2014 from C. difficile infections in the U.S.
The present therapies to treat C. difficile infection are plagued by significant CDI recurrences underscoring the need for new treatments.
IMM- 529 Drug Development Plan
The company intends to develop IMM-529 as a drug to prevent recurrent Clostridium difficile Infection. The drug development plan would be implemented as per the following steps:
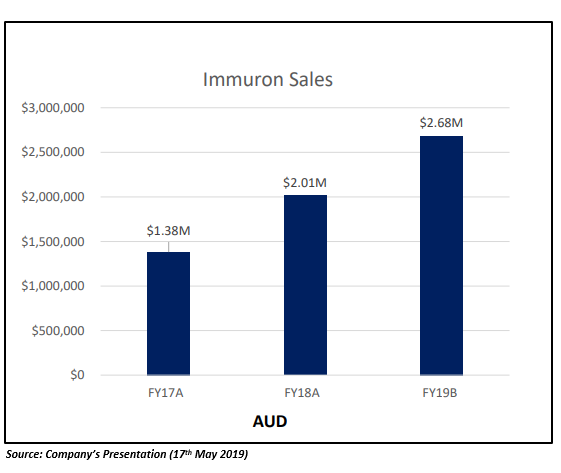
The company released its third quarter FY19 sales results on 15th April 2019. The company witnessed a 66 per cent YoY growth in worldwide sales during the third quarter of FY19. The sales of the Travelan® surged in Australia in Q3, growing 73% YoY. The company also received its first purchase order for Canada during Q3 FY19.
The companyâs stock closed the dayâs trade at AUD 0.185 (on 17th May 2019). The stock has not performed well during the last few months. It has performed in the negative territory in the previous one month, generating a negative return of 22.92 per cent.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. Kalkine Media does not in any way endorse or recommend individuals, products or services that may be discussed on this site. Our publications are NOT a solicitation or recommendation to buy, sell or hold. We are neither licensed nor qualified to provide investment advice.




