Clinical stage Australian immuno-oncology company, Imugene Limited (ASX:IMU) is set to kickstart two clinical trials with B-cell immunotherapy - PD1-Vaxx and oncolytic virotherapy CF33 this year. Recently, Imugene presented presentations on the clinical plan for both these trials at the American Association for Cancer Research (AACR) 2020 Virtual Annual Meeting.
Below are the titles of the abstract presentations presented at the event:
- An Open Label, Multi Center, Dose Escalation/Expansion, Phase 1 Study of IMU-201 (PD1-Vaxx), a B-Cell Immunotherapy, in Adults with Non-Small Cell Lung Cancer (NSCLC) - Presented by Dr Anthony Good, Imugene’s Vice President of Clinical Research
- A First-in-Human Phase 1 Ascending, Multiple Dose Safety and Tolerance Study of VAXinia (CF33 + hNIS), a Novel Chimeric Oncolytic Poxvirus, Administered Intratumorally or Intravenously as a Monotherapy or in Combination with an Immune Checkpoint Inhibitor (ICI) in Adult Patients with Mixed Advanced Solid Tumors (MAST) - Presented by Dr Seymour Fein, Consulting Medical Director to Imugene
PD1-Vaxx (IMU-201) Phase 1 NSCLC Study
Imugene is developing PD1-Vaxx to stimulate the production of anti-PD-1 antibodies via active immunisation of patients with a peptide epitope designed to induce polyclonal antibodies against PD-1.
This vaccination strategy may afford the possibility of producing an enduring immune response, inducing high-affinity anti-PD-1 antibodies that act as antagonists to receptor signalling that prevents T-cell responses to tumors.
The Company’s PD1-Vaxx Phase 1 Proof of Principle study is an open-label dose escalation/dose expansion study of IMU-201, divided into two parts:
Part-1: Dose Escalation Study
- Dose escalate IMU-201 as monotherapy and in combination with SOC (Standard of Care) treatment to assess immunogenicity, safety and tolerability and evaluate the optimal biologic dose (OBD) of IMU-201.
Part 2: Expansion of OBD
- Expand the number treated with IMU-201 at OBD in combination with SOC treatment.
The Company has planned to enrol a total of about 30 adult patients aged 18 years with NSCLC expressing PD-L1 in the Phase 1 PD1-Vaxx study at sites in Australia and North America. The first patient is expected to be dosed in Q3-4, 2020.
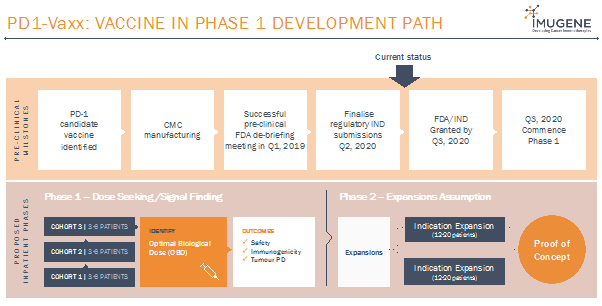
Source: Company’s Presentation (Wholesale Investors Feb 2020)
Objectives
As per the abstract presentation , the key objectives of the PD1-Vaxx Phase 1 study include:
- Assess the tolerability/safety and immunogenicity of IMU-201 as monotherapy and in combination with SOC treatment.
- Identify OBD of IMU-201 as monotherapy and in combination with SOC treatment.
- Assess changes in biochemical, immunological and additional radiological markers of tumor progression in patients treated with IMU-201 as monotherapy and in combination with SOC treatment.
- Evaluate changes in cellular and humoral immunogenicity data comprising PD-1 specific antibodies (IgG, IgM), vaccine-specific cytokine levels and regulatory and effector T and B cells.
Also Read! Imugene Announced Completion of GMP manufacturing and Toxicology Studies for PD1-Vaxx
VAXinia (CF33-hNIS) Phase 1 MAST Study
Imugene has also planned to conduct a Phase 1, open-label, dose escalation study in adult patients suffering from selected advanced or metastatic solid tumors to assess the safety and tolerance of CF33-hNIS, administered intratumorally (IT) or intravenously (IV).
The tumor types eligible for CF33-hNIS IT administration include advanced or metastatic melanoma, triple negative breast cancer (TNBC) with superficial lesions and head and neck squamous cell cancer with superficial lesions.
On the other hand, the tumor types eligible for CF33-hNIS IV administration include advanced or metastatic melanoma with no accessible lesions, bladder/urothelial cancer, TNBC, NSCLC, colorectal cancer, gastroesophageal adenocarcinoma, head and neck squamous cell cancer and renal cell carcinoma.
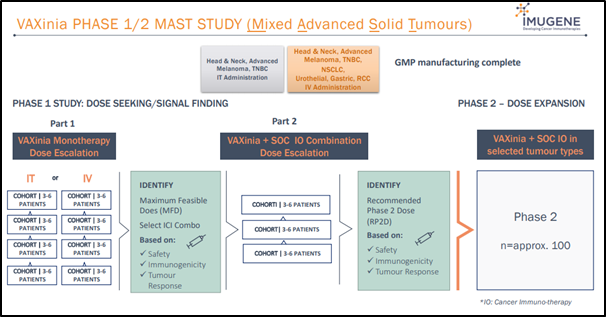
Source: Company’s Presentation (Wholesale Investors Feb 2020)
Objectives
As per the abstract presentation, the key objectives of VAXinia Phase 1 MAST study include:
Primary Objectives
- Safety of CF33-hNIS, IT or IV, as monotherapy or in combination with ICI.
- Determine RPL2D as monotherapy or in combination with ICI.
Secondary Objectives
- Evaluate anti-tumor activity of CF33-hNIS as monotherapy or in combination with ICI using ORR, PFS, 6-month PFS.
- Assess viral titers in serum, urine, oral and rectal swabs by VPA and PCR.
- Assess tumor infection with CF33-hNIS by hNIS-based imaging.
Exploratory Objectives
- Evaluate antiviral immune activation i.e. cytokines and lymphocyte subsets.
- Assess biopsies of selected tumors for histology, immune cell infiltration and expression of PD-1, PD-L1 and CTLA-4, immunohistochemistry staining.
Interesting Read! Imugene Announces Completion of Clinical Grade GMP Batches by CF33 Oncolytic Virus Constructs
Way Ahead
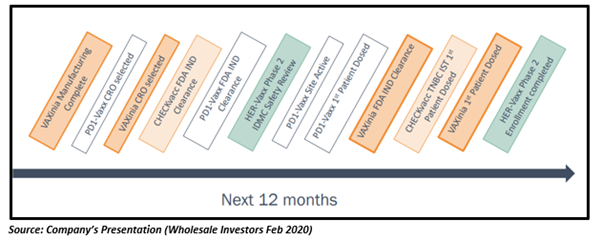
IMU last traded at $0.030, up 3.45% on 13 May 2020.
Must Read! Take a Quick Peek into Imugene’s Q1 2020 Operational Highlights



