1. Current State and Demographic Trends of Medically Underserved Populations in Rare Disease Research in the United States.
2. A Framework for Inclusive and Accessible Clinical Research in Rare Diseases.
The manuscripts were developed through a year-long collaboration of RDDC’s Research & Clinical Trials Working Group and IndoUSrare, with analytical contributions from academic, patient-advocacy, and industry partners. Both manuscripts are undergoing peer review at a health services research journal, and preprints are freely available on medRxiv.
Speaking about the research, Jenifer Ngo Waldrop, Executive Director of RDDC, says, “These data confirm what patients have told us for years: the rare-disease research enterprise is not reaching everyone who needs it. With this framework, we are giving sponsors and regulators clear next steps to close the gap.”
Harsha K. Rajasimha, PhD, Founder & Executive Chair of IndoUSrare, highlights the pressing need for collaborations to drive reforms for research and clinical trials for rare diseases, “Equity is not a checkbox. It requires infrastructure, community trust, and accountability."
The first preprint, “Current State and Demographic Trends of Medically Underserved Populations in Rare Disease Research in the United States,” presents a thirty-year review of literature and clinical trial data, revealing that fewer than 12 percent of U.S.-based rare disease studies report basic participant demographics such as race, ethnicity, income, or rural status. This lack of transparency has rendered many medically underserved populations largely invisible in clinical research, perpetuating disparities in diagnosis, treatment access, and therapeutic development.
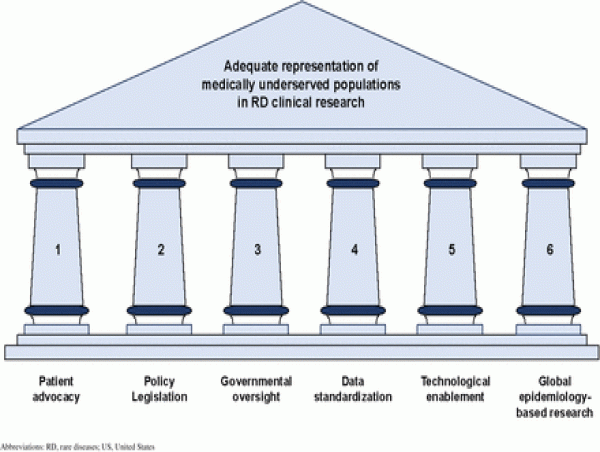
The second manuscript, “A Framework for Inclusive and Accessible Clinical Research in Rare Diseases,” proposes a practical model to address these gaps. Drawing from successful approaches in pediatric, oncology, and disability-focused research, the framework outlines a six-pillar framework that focuses on community partnership, inclusive protocol design, tech-enabled access, and transparent reporting. These pillars include (1) Patient Advocacy, (2) Policy Legislation, (3) Government Oversight, (4) Data Standardization, (5) Technological Enablement, and (6) Global Epidemiology-based Research. Designed to be scalable and adaptable, the framework equips sponsors, research institutions, and regulators with concrete steps to make rare disease trials more accessible to all populations.
Contributors to the research emphasised the urgency of the findings. “Contributing to the demographic trends manuscript was important to the patient-reported outcomes work I do, as there is limited data to cite and highlight that significant gaps continue to exist in medically underserved populations in clinical trial samples,” says Cindy Umanzor Figueroa, Manuscript Contributor and RDDC member. Harriet Tunu Baraka, Manuscript Contributor and Reviewer, Data Analyst, and Interpreter, echoes this, "Being part of this project reinforced the urgency of equitable representation in rare disease research. By shedding light on who is missing from the data, we move closer to designing research that truly serves all communities."
"Our analysis shows that fewer than 12% of rare disease studies in the U.S. report even the most basic demographic details—leaving entire communities, including the Indian diaspora, invisible in the data. At IndoUSrare, we are committed to changing that by not only identifying these gaps but by offering a practical, inclusive framework that global stakeholders can act on today", says Dr. Rajasimha.
Researchers, biopharma executives, regulators, patient leaders, and media are encouraged to join the dialogue to help refine the proposed framework ahead of peer review and publication. The authors will host open forums at two major conferences this June:
1. BIO International Convention - June 16-19, at the Boston Convention & Exhibition Center in Boston, MA,
2. DIA Global Annual Meeting - June 15-19, at the Walter E. Washington Convention Center in Washington, DC.
3. Indo US Bridging RARE Summit - Nov 2-4, at Hylton Performing Arts Center, Manassas, VA
IndoUSrare invites stakeholders to continue this critical conversation and drive actionable change. "We look forward to connecting with global stakeholders at BIO 2025 and DIA Global 2025 next week, and at the Indo US Bridging RARE Summit 2025, an international convening that aims to directly address the representation gaps identified in the research, with a special focus on one of the most underserved and overlooked populations in rare disease research worldwide: the Indian diaspora. Dr. Rajasimha states, "IndoUSrare is proud to partner with RDDC among other global partners to bring together researchers, policymakers, industry leaders, and patient advocates to forge cross-border collaborations that reflect the diversity of the global rare disease community."
About the Rare Disease Diversity Coalition (RDDC)
RDDC is an initiative launched by the Black Women’s Health Imperative, a 501(c)3, to address the extraordinary challenges faced by historically underrepresented populations with rare diseases. RDDC brings together rare disease experts, health and diversity advocates, and industry leaders to identify and advocate for evidence-based solutions to reduce racial disparities in the rare disease community. Learn more at rarediseasediversity.org.
About Indo US Organization for Rare Diseases (IndoUSrare)
IndoUSrare is a 501(c)(3) nonprofit accelerating cross-border collaborations, patient advocacy, and inclusive research to address the unmet needs of 400 million people living with rare diseases worldwide. Learn more at indousrare.org
These preprints have not yet undergone peer review. Findings should not guide clinical practice until verified.
Media Contacts
For RDDC:
Name: Derika Crowley | Email: [email protected] | Phone: +1 267-964-3386
Nisha Venugopal
Indo US Organization for Rare Diseases
+1 540-239-0465
[email protected]
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()