Japan has long prioritized innovation in regenerative and personalized medicine. In recent years, CAR-T therapy and advanced treatment involving a patient’s re-engineered immune cells has moved from being a niche intervention to a beacon of hope for patients with relapsed or refractory cancers. The expanding pipeline of CAR-T products, growing clinical success, and strong government backing are shaping a thriving and competitive market.
To Download Sample Report: https://datamintelligence.com/download-sample/japan-car-t-cell-therapies-market
Key Growth Drivers
High Burden of Blood Cancers
With an aging population and increasing incidence of hematologic malignancies, Japan faces rising pressure to adopt more targeted, effective treatments. CAR-T therapies, showing unprecedented remission rates, are becoming the frontline solution for otherwise intractable cases.
Regulatory Acceleration
Japan’s regulatory body has adopted a supportive stance, with fast-track designations and conditional early approvals for innovative therapies. This has made Japan one of the fastest countries globally to review and authorize advanced cell therapies.
Academic and Industry Collaboration
Prestigious universities and biotech firms are co-developing CAR-T therapies, optimizing production protocols, and localizing manufacturing to improve supply chains and reduce treatment delays.
Increased R&D and Clinical Trials
Clinical trials for both hematologic and solid tumors are actively underway in Japan. As real-world evidence continues to support efficacy and safety, broader payer coverage and physician confidence are boosting uptake.
Government Investment and Policy Push
Japan has committed to becoming a global biotech hub, including initiatives aimed at scaling regenerative medicine platforms and enhancing accessibility of life-saving therapies like CAR-T.
Market Challenges
High Treatment Costs
The cost of developing and administering CAR-T therapy remains a significant barrier. Individual treatments can exceed hundreds of thousands of dollars, limiting widespread accessibility.
Limited Treatment Centers
Only a handful of hospitals in Japan are equipped to handle the full CAR-T therapy cycle, from apheresis to infusion and post-treatment monitoring. Geographic concentration of expertise leads to access gaps in rural areas.
Supply Chain and Production Bottlenecks
Autologous CAR-T therapies (derived from the patient’s own cells) require complex logistics, making it difficult to rapidly scale.
Side Effects and Specialized Care Needs
CAR-T can trigger serious immune responses, including cytokine release syndrome (CRS). Specialized monitoring and immediate intervention capabilities are required, which limits adoption to only high-tier medical centers.
Key Players:
Gilead Sciences, Inc.
Bristol Myers Squibb Company
Johnson & Johnson Services, Inc.
Novartis AG
Emerging Players:
Takara Bio Inc
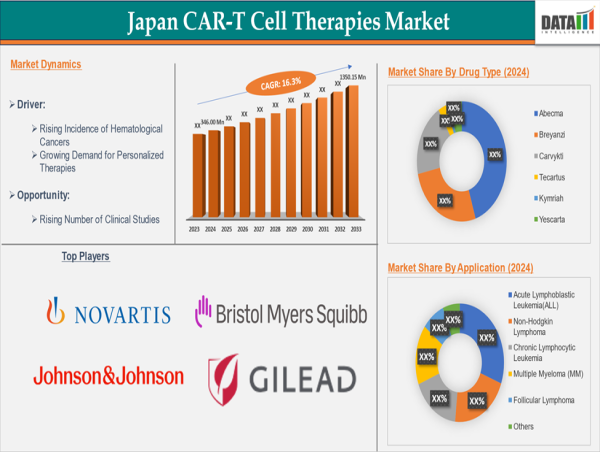
Market Segmentation
By Therapy Type: Autologous CAR-T Cell Therapy, Allogeneic CAR-T Cell Therapy.
By Drug Type: Abecma, Breyanzi, Carvykti, Tecartus, Kymriah, Yescarta.
By Target Antigen: CD19, BCMA (B-cell maturation antigen), CD20, CD22, CD30, Others.
By Application: Acute Lymphoblastic Leukemia (ALL), Non-Hodgkin Lymphoma, Chronic Lymphocytic Leukemia, Multiple Myeloma (MM), Follicular Lymphoma, Others.
Regional Outlook
Tokyo, Osaka, and Nagoya form the major hubs for CAR-T therapy delivery in Japan. However, growing investments in local biotech ecosystems in regions such as Hokkaido and Kyushu are beginning to decentralize the market. Local manufacturing partnerships are emerging to reduce lead times and ensure more equitable national access.
Latest News: United States CAR‑T Cell Therapy Developments
The U.S. remains the global leader in CAR-T therapy innovation and commercialization. In 2025, significant developments are setting new benchmarks for the industry:
Breakthroughs in Solid Tumors
New clinical data from U.S.-based trials show promising results for CAR-T therapies targeting solid tumors like glioblastoma and gastric cancers—fields previously thought resistant to CAR-T.
FDA Approvals and Innovation Expansion
The FDA recently approved new CAR-T therapies that feature next-generation safety switches and more targeted delivery, enhancing both efficacy and patient outcomes.
Mergers and Strategic Investments
U.S. pharmaceutical giants are acquiring CAR-T start-ups and expanding their cell therapy divisions, reflecting confidence in long-term scalability and profitability of the CAR-T model.
Latest News: Japan CAR‑T Therapy Highlights
Japan has also seen transformative developments in its CAR-T therapy landscape in early 2025:
Gilead’s Expansion of Yescarta
Gilead Sciences is rapidly expanding the availability of Yescarta, having partnered with Japanese hospitals to expand training and infusion site accreditation, ensuring broader access across the country.
Automation of CAR-T Manufacturing
Kyoto University’s Center for iPS Cell Research and Application (CiRA) has launched a new facility to automate the production of autologous immunotherapies, potentially lowering costs and increasing patient throughput.
Homegrown CAR-T Trials Enter Advanced Phases
Japanese biotech companies have successfully entered late-stage trials for domestically developed CAR-T therapies, targeting both hematologic and early-stage solid tumors, marking a major step toward therapeutic independence.
Policy Reform Supporting Innovation
Japan’s Ministry of Health is updating reimbursement structures for advanced therapies, including partial coverage schemes to lower patient burden and incentivize providers to adopt new technologies.
Strategic Outlook
Japan is well-positioned to become a regional leader in CAR-T therapies, supported by:
Strong public–private partnerships in R&D
Regulatory frameworks that balance speed with safety
Growing clinical success stories fueling adoption
Expansion of local manufacturing and automation technologies
Government policies prioritizing innovation and access
As CAR-T evolves from being a "last-resort" option to a frontline treatment, Japan’s sustained investments in infrastructure, workforce training, and patient access will play a crucial role in shaping the next era of personalized cancer care.
Looking For A Detailed Full Report? Get it here: https://datamintelligence.com/buy-now-page?report=japan-car-t-cell-therapies-market
Stay informed with the latest industry insights-start your subscription now: https://www.datamintelligence.com/reports-subscription
Related Reports:
Cell Therapy Market
CAR-T Cell Therapy Market
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()