Summary
- Nasal vaccine administration has tremendous potential because of an organised immune system, high compliance, and low cost.
- US-based Codagenix Inc and the Serum Institute of India have started Phase 1 clinical study of COVI-VAC.
- Bharat Biotech is set to commence Phase 1 clinical study of its nasal COVID-19 vaccine in February or March.
- Nasal COVID-19 vaccine by Lancaster University has demonstrated significant results in pre-clinical testing.
The intramuscular route has been thought of as the ultimate way to administer a vaccine. However, intranasal vaccines have multiple advantages of their own, including ease of self-administration and systematic immunity.
The nasal route has outstanding potential for vaccination due to an organised mucosal immune system. Some of the key advantages of the intranasal route are:
- A nasal vaccine is much less invasive for the recipient compared to an intramuscular injection.
- For those who are afraid of needles or have blood clotting-related disorders, nasal vaccine proves to be a suitable alternative.
- High compliance (Suitable for both adults and children).
- A nasal spray vaccine is easy to administer besides obviating the need of medical consumables (needles, syringes etc.).
- A nasal vaccine can offer a low-cost option for the developing world as mass manufacturing of the intranasal vaccine can be simplified using the exiting production technology for flu vaccines.

Source: Copyright © 2021 Kalkine Media Pty Ltd.
However, despite the advantages of intranasal COVID-19 vaccines and encouraging findings observed in animal studies, human clinical trials are still required to determine the safety and efficacy of nasal spray vaccine against SARS-CoV-2.
With this backdrop, let us zero in on a few updates in the COVID-19 nasal vaccine development space:
Phase 1 trial of COVI-VAC nasal vaccine commences
For COVI-VAC nasal vaccine, Phase 1 clinical trial has been kicked off by US-based Codagenix Inc. and Serum Institute of India. They have ticked off a crucial milestone as the first patient has been dosed for the clinical trial. COVI-VAC is a live attenuated, single-dose, intranasally-administered vaccine against SARS-CoV-2. The nasal vaccine can offer a thorough immune response compared to other COVID-19 vaccines.
Moreover, COVI-VAC was developed with the Synthetic Attenuated Virus Engineering (SAVE) platform of Codagenix. This platform uses synthetic biology for virus gene re-coding into safe and stable vaccines.
COVI-VAC is designed to offer a safe and more robust immune response along with enduring cellular immunity against coronavirus compared to other vaccines.
- Robert Coleman CEO of Codagenix, commented:
Additionally, COVI-VAC can also address several significant logistical challenges to immunisation against novel coronavirus worldwide. Being an intranasal vaccine, COVI-VAC can be produced at large scale, and it can be administered easily with minimum instructions in a mass vaccination campaign.
Bharat Biotech to begin Phase 1 clinical trial of BBV154
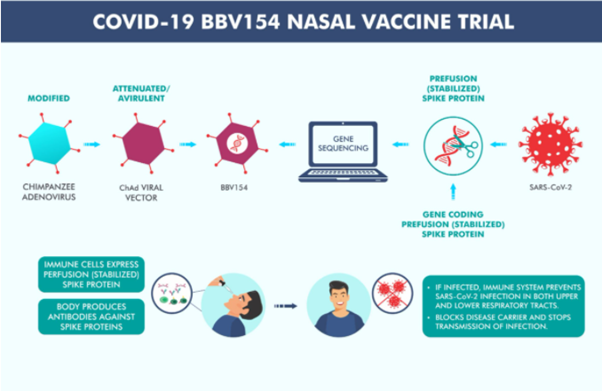
Along with Covaxin, Bharat Biotech is developing another COVID-19 vaccine, an intranasally administered vaccine named BBV154.
BBV154 is a novel intranasal, adenovirus vectored vaccine against SARS-CoV-2 virus. The Company states that the vaccine candidate ChAd-SARS-CoV-2-S shows protective efficacy in mice and hamster.
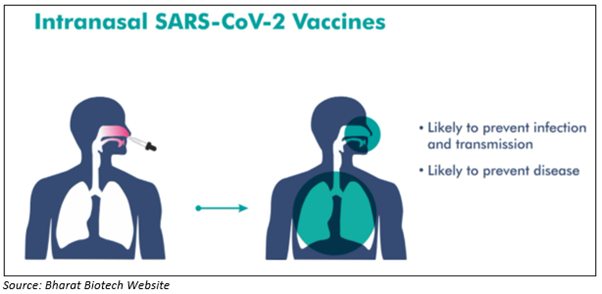
On immunisation with a single dose of ChAd-SARS-CoV-2-S, animals (mice and hamsters) demonstrated superior protection against coronavirus. After SARS-CoV-2 challenge, viral clearance was seen in both lower as well as upper airways.
Hence, intranasal immunisation of ChAd-SARS-CoV-2-S can generate an immune response in the nose, one of the virus’s entry points. Therefore, it protects against infections and further spread.
Phase 1 clinical trials to commence in Feb-March 2021
Bharat Biotech disclosed that the Company would start Phase 1 clinical trials of BBV154 for the coronavirus during February-March this year. The Company stated that the pre-clinical testing of BBV154 was completed for immunogenicity, toxicology, and challenge studies. Pre-clinical studies have been conducted in India and the US.
Source: Bharat Biotech Website
On 19 January 2020, CDSCO’s Subject Expert Committee (SEC) recommended the Indian biotech Company’s intranasal vaccine for Phase 1 clinical trials against the novel coronavirus. However, the final decision would be taken by the DCGI (Drugs Controller General of India).
The Hyderabad-based player disclosed that Phase 1 clinical trials would be conducted in Saint Louis University's Vaccine and Treatment Evaluation Unit.
Chairman of Bharat Biotech Krishna Ella said:

Mr Ella also added that an intranasal vaccine would be simple to administer and reduce medical consumables usage (needles, syringes, etc.). These advantages will slash the overall expenses of the vaccination drive significantly.
Lancaster University’s nasal COVID-19 vaccine
Researchers at Lancaster University have engineered a COVID-19 vaccine for intranasal administration.
The researchers administered two doses of the intranasal vaccine to the animals which elicited antibodies as well as T-cell responses, effective enough to neutralise coronavirus. Moreover, the vaccinated rodents also witnessed a substantial reduction in lung pathology, inflammation, and clinical indication.
Lancaster University’s vaccine is based on a common poultry virus named the Newcastle Disease Virus (NDV). This virus can replicate in humans, but it is not dangerous. The scientists engineered the Newcastle Disease Virus to produce the spike proteins of the novel coronavirus, tricking the body to generated increased immune response against the virus.
Virologist Dr Muhammad Munir commented:

Researchers at Lancaster University anticipate that this intranasal vaccine could offer a low-cost alternative for the world. The manufacturing of this vaccine can be increased by utilising already existing global infrastructure, currently in use for influenza virus vaccines.
The successful development of an intranasal vaccine will offer the most economical vaccine supply across the globe.
ALSO READ: Lab tests confirm BioNTech (NASDAQ:BNTX), Pfizer vaccine effective against new Covid-19 variants