A meeting was also held in Germany to announce the results of the international pharmacokinetic similarity clinical (Phase 1) study
BERLIN, Jan. 12, 2024 /PRNewswire/ -- Boan Biotech recently announced that subject enrollment for the international multicenter comparative clinical (Phase 3) study of the company's Denosumab Injections (BA6101 and BA11021) has been completed. This study is being conducted simultaneously in Europe, the United States and Japan. A meeting was also recently held in Germany to announce the results of the pharmacokinetic similarity clinical (Phase 1) study for BA6101. Dr. Dou Changlin, President of R&D and Chief Operating Officer of Boan Biotech, and Dr. Zhou Ming, Chief Medical Officer of Boan Biotech, attended the meeting along with Dr. Rainard Fuhr from Parexel International GmbH, Principal Investigator of the Phase 1 clinical study of BA6101. Together with other clinical investigators, they discussed the results of this study and the application of Boan Biotech's Denosumab Injections in international markets.

Dr. Dou Changlin (5th from left in the front row), Dr. Zhou Ming (4th from left in the front row) and clinical investigators of the Phase 1 study
BA6101 and BA1102 are biosimilars to Denosumab Injection original drugs Prolia® and Xgeva® respectively. Prolia is widely used for treating osteoporosis around the world, whereas Xgeva is used globally for the treatment of diseases such as multiple myeloma and bone metastasis of solid tumors, giant cell tumors of bone, and hypercalcemia.
In November 2022, BA6101 (trade name: Boyoubei® in Chinese) was launched in China firstly, to become China's first locally developed Denosumab Injection. The drug received positive feedback from doctors and patients on its clinical application. In March 2023, the biologics license application (BLA) of BA1102 was also accepted by China's National Medical Products Administration (NMPA) and will be approved soon.
With the first-mover advantage for denosumab in China, Boan Biotech is actively driving the international clinical studies and registration. In 2020, the Investigational New Drug (IND) application for BA6101 was approved in the United States and Europe, and the Phase 1 clinical study (pharmacokinetic similarity study) was completed in Europe and the results were announced at this meeting. In 2022, the Phase 3 clinical study (comparative clinical study) was proceed in Europe, the United States, and Japan and has completed subject enrollment recently.
At the meeting in Berlin, Germany, Dr. Fuhr as the Phase 1 study's Principal Investigator discussed the results of the study in detail with the team from Boan Biotech. This was a randomized, double-blind, single-dose, three-arm, parallel-group study designed to demonstrate the PK similarity between BA6101, US-Prolia and EU-Prolia and to evaluate the safety, tolerability, immunogenicity and PD of BA6101 compared with US-Prolia and EU-Prolia. The results shown that all three pairwise comparisons met the pre-specified acceptance criteria of bioequivalence for PK and PD and demonstrated similarity in PK, PD, safety and immunogenicity.
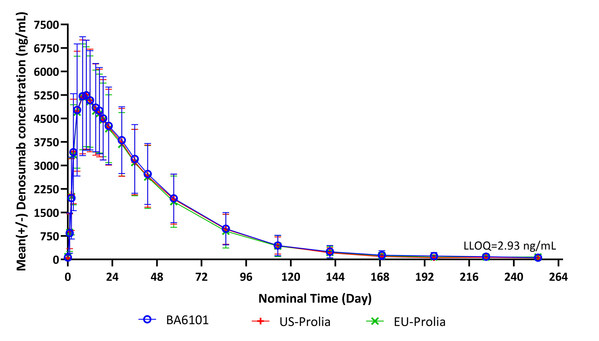
Mean (+/- SD) Denosumab Concentration Time Profile in Linear Scale (Pharmacokinetic Concentration Analysis Set)
Dr. Fuhr said at the meeting: "Denosumab is used globally and playing an important role in treating related diseases. Developing denosumab biosimilars is important to meeting patient needs in clinical practice. We're very excited to see the data presented by Boan Biotech, showing high-quality progress of the international clinical studies for BA6101, and we hope these two products can be launched as soon as possible, to provide quality treatments for patients and increase the accessibility of denosumab for them."
The international comparative clinical (Phase 3) study of Boan Biotech's denosumab is being conducted simultaneously in Europe, the United States, and Japan. This is an international multicenter, randomized, double-blind, parallel and reference-controlled clinical study to compare BA6101 with the reference drug Prolia for efficacy, safety, pharmacokinetics, and immunogenicity. According to the Scientific Considerations in Demonstrating Biosimilarity to a Reference Product issued by the U.S. Food and Drug Administration (FDA)2, the Guideline on Similar Biological Medicinal Products issued by the European Medicines Agency (EMA)3, and the Guideline for the Quality, Safety, and Efficacy Assurance of Follow-on Biologics issued by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA)4, as well as the opinions expressed by FDA, EMA, and PMDA after talking with Boan Biotech, after the completion of the comparative clinical study of BA6101, the BLA for BA6101 and BA1102 can be made in Europe, the United States, and Japan for all the indications approved for Prolia and Xgeva.
Dr. Dou Changlin, President of R&D and Chief Operating Officer at Boan Biotech, said: "Publicly available data shows that Prolia and Xgeva achieved global sales of $3.63 billion and $2.01 billion respectively in 2022. It can be anticipated that the size of international markets for biosimilars of denosumab will be substantial. We will strictly follow the Good Clinical Practice (GCP) requirements to conduct the international Phase 3 study, and will also continue to build a system complying with the current Good Manufacturing Practices (cGMP) required by the authorities in Europe, the United States, and Japan, to prepare for submission of the marketing authorization applications in those jurisdictions."
About Boan Biotech
Boan Biotech (6955.HK) is a fully-integrated biopharmaceutical company developing, manufacturing, and marketing biologics, with a focus on oncology, autoimmune diseases, ophthalmology, and metabolic diseases. The company's drug discovery activities revolve around multiple platforms: Human Antibody Transgenic Mouse and Phage Display Technology Platform, Bispecific T-cell Engager Technology Platform, ADC Technology Platform and Cell Therapy Platform.
Boan Biotech operates across the entire value chain of the industry covering antibody discovery, cell line development, upstream and downstream process development, analytical and bio-analytical method development, technology transfer, non-clinical research, clinical research, regulatory affairs and registration, and commercial production. In the cell therapy field, Boan Biotech focuses on a new generation of enhanced and regulated CAR-T technology, developing safer, more effective, and affordable treatments for patients.
Boan Biotech's portfolio includes two commercial products. Its pipeline includes multiple novel biologics as drug candidates protected for their international intellectual property rights and a number of biosimilar candidates. In addition to China, the company is also developing biopharmaceutical products in overseas markets, including the U.S. and the EU. With a differentiated portfolio and well-established commercial capabilities, Boan Biotech operates across the industry's value chain from research and development to manufacturing and commercialization. This has laid a solid foundation for its long-term, high-quality growth.
1. Note: BA6101 and BA1102 were previously coded-named LY06006 and LY01011 respectively. |
2. FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. April 2015 |
3. EMA. Guideline on similar biological medicinal products. CHMP/437/04 Rev 1. 2015 |
4. PMDA. Guideline for the quality, safety, and efficacy assurance of follow-on biologics. 2009 |



