VANCOUVER, BC, April 2, 2024 /PRNewswire/ -- WAT Medical Enterprise achieves a major milestone: HeadaTerm 2 receives the OTC clearance from the U.S. Food and Drug Administration (FDA), making it one of the only wearable anti-migraine devices that is available without a prescription in the U.S.
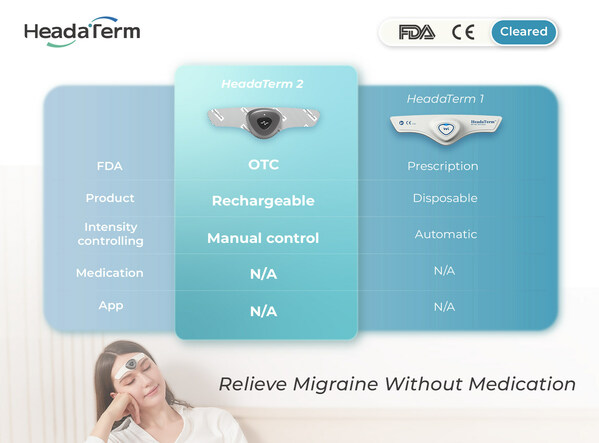
Using neuromodulation technology, HeadaTerm 2 releases targeted electrical impulses to increase pain tolerance of its users. It is FDA-cleared for the preventative treatment of migraine headaches.
The device has been clinically proven to be safe and effective at reducing migraine symptoms; according to the study published in the American Journal of Emergency Medicine, it can be up to 27%[1] more effective at relieving migraine symptoms than oral medications. Additional clinical studies featuring HeadaTerm 2 will commence in 2024 at world-renowned research institutions including University of Toronto, McMaster University, and Ohio State University.
HeadaTerm 2 is a testament of WAT Medical's continued commitment to providing safe, effective, and user-friendly innovative medical devices at the most competitive price. HeadaTerm 1 was the world's first disposable wearable anti-migraine device. It provided an affordable solution to a debilitating condition that affects nearly 40 million people in the U.S. HeadaTerm 2 is a rechargeable device that features adjustable intensity setting and will be the most affordable device of its kind.
With its streamlined design, HeadaTerm 2 requires no app, enhancing its user-friendliness by allowing all controls to be managed with just one button, ensuring ease of operation. This innovative approach simplifies the user experience, eliminating the need for complex installations or additional software. During extensive usability testing, consumers unanimously praised its intuitive interface and seamless functionality, regarding it as simply the best solution for managing migraines effectively.
WAT Medical plans to support headache patients, specifically veterans, through strategic partnerships and donations through the VHA Headache Centers of Excellence Program. HeadaTerm 2 will appear at the 66th Annual Scientific Meeting in June 2024 hosted by American Headache Society. The device will be available without a prescription at www.emeterm.com and on Amazon in April 2024.
[1] Hokenek N M, Erdogan M O, Hokenek U D, et al. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomize controlled trial[J]. The American Journal of Emergency Medicine, 2021, 39: 80-85. |