 |
First and only contact force pulsed field ablation (PFA) system designed to transform treatment for millions with life-threatening ventricular arrhythmias.
Preliminary data of VCAS study will be presented at HRS 2024 in Boston, Mass.
CARDIFF-BY-THE-SEA, Calif., May 9, 2024 /PRNewswire/ -- Field Medical,™ Inc., a pioneer in pulsed field cardiac catheter ablation technology, today announced the initiation of its first-in-human study for the FieldForce™ Ablation System at Na Homolce Hospital in Prague, Czech Republic. The Ventricular Catheter Ablation Study, VCAS, will enroll up to 60 patients at up to 5 sites world-wide. The VCAS investigational study is a critical step towards demonstrating safety and performance of the FieldForce™ Ablation System, developed specifically to address the limitations of existing pulsed field ablation (PFA) and radiofrequency (RF) ablation systems, including a more time efficient treatment protocol for the treatment of ventricular arrhythmias. Preliminary results will be presented on May 16, 2024, at the Heart Rhythm Scientific Sessions in Boston, Mass.
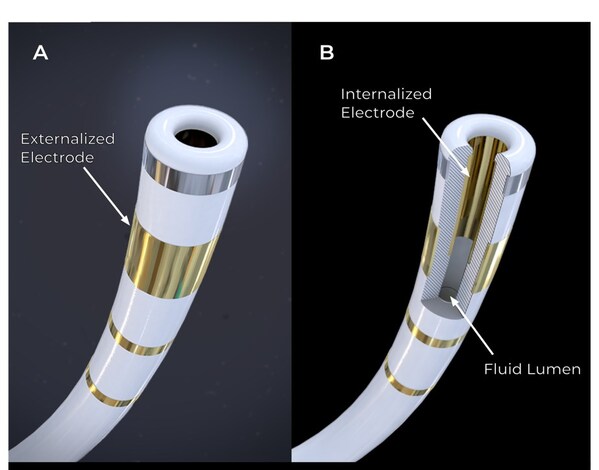
Field Medical’s FieldForce™ Catheter tip (A). The FieldBending™ technology utilizes an innovative internalized electrode within a fluid lumen seen in the cutaway (B). Voltage is delivered between the external and internal electrodes resulting in desirable electric field characteristics. The FieldForce™ Catheter is the first and only contact force PFA catheter optimized for the ventricles.
"The FieldForce Ablation System, as the first contact force PFA system for ventricular ablation, is demonstrating significant promise early in our trials," said Vivek Reddy, MD, Director of Electrophysiology for the Mount Sinai Health System. "This is my first experience with a technology capable of achieving rapid complete transmural left ventricular (LV) ablation, offering highly targeted treatment options and an efficient workflow. Although these results are quite preliminary, the potential of this system to revolutionize catheter ablation of complex ventricular arrhythmias is profound, potentially surpassing the current standard of care. The scientific community will benefit from the comprehensive results from the completed study."
PFA Adoption and Transition from Atrial to Ventricular Arrhythmias
PFA has revolutionized the treatment of atrial fibrillation (AF) over the past decade, thanks to pioneering work by Field Medical CEO Steven Mickelsen, MD, founder of Farapulse which was subsequently acquired by Boston Scientific. His development of PFA technology marked a significant shift in cardiac electrophysiology, enhancing safety and reducing procedure times. This innovation set a new standard in the industry, leading to widespread adoption and the transformation of ablation practices. With PFA's proven success in atrial settings, Field Medical is now poised to extend these benefits to ventricular arrhythmias, signaling a pivotal evolution in the management of complex ventricular cardiac conditions.
Ventricular Arrhythmia Burden
It is estimated that ventricular arrhythmia impact over 6 million patients in the US and Europe. Ventricular arrhythmias, including ventricular tachycardia (VT) and premature ventricular contractions (PVCs), and pose a substantial risk, often leading to sudden cardiac death if not adequately managed. Current ventricular catheter ablation methods involve lengthy procedures and significant risks associated with thermal ablation, like conventional RF ablation. Field Medical's PFA technology aims to address these challenges by providing the first and only contact force PFA catheter optimized to work in the ventricle. This novel technology may enable predictable, time efficient, and safe treatment option. FieldBending™ takes advantage of non-intuitive physics to deliver intense, yet brief, electric fields designed to safely reduce procedure times dramatically.
"Our vision at Field Medical is to transform VT ablation into a widely available, one-hour outpatient procedure with improved safety outcomes," said Steven Mickelsen MD, CEO of Field Medical. "With the promising early data for our VCAS study, in much the same way that PFA transformed AF, the FieldForce Ablation System is optimized to transform how ventricular arrhythmias are treated, offering hope to millions of patients worldwide. With the strong investor support, renown physician engagement and the groundbreaking potential of our technology, we are set to redefine the standards of cardiac ablation once again."
About VCAS Study
Field Medical's VCAS Study is a pivotal segment of a prospective safety and feasibility study, assessing the innovative FieldForce Ablation System in patients with ventricular arrhythmias. The VCAS-I group focuses on individuals suffering from ventricular tachycardia (VT), while the VCAS-II group targets those experiencing frequent premature ventricular complexes (PVCs). This study is instrumental in evaluating the performance of Pulsed Field Ablation (PFA), thereby potentially reducing procedural time compared to traditional Radiofrequency (RF) ablation. The trial aims to demonstrate that PFA can safely treat and improve overall outcomes for patients with challenging ventricular arrhythmias. For study information, visit clinicaltrials.gov NCT: NCT06203262.
About Field Medical,™ Inc.
Founded in 2022, Field Medical is at the forefront of developing second-generation pulsed field ablation (PFA) technologies, designed to meet the technical and safety challenges of modern cardiac ablation procedures. Under the leadership of Dr. Steven Mickelsen, CEO, a globally recognized expert in pulsed electric field technology, the company has pioneered the proprietary FieldBending™ technology. This innovation was developed to concentrate therapeutic effects and reduce unwanted far-field effects, with the potential to improve patient outcomes while simplifying the clinical experience for physicians. Field Medical focuses on expanding the applications of PFA beyond atrial fibrillation (AF) to address complex ventricular arrhythmias—a condition affecting over 6 million people in North America and Europe alone, often leading to sudden cardiac death, heart failure and significant clinical symptoms if not managed effectively. The company's comprehensive product portfolio, including the FieldForce™ Catheter, FieldForce™ Ablation System, and the FieldFlex™ Sheath, is specifically optimized to work in complex ventricular environments. For more information, visit www.fieldmedicalinc.com and follow us on LinkedIn and X.
The FieldForce™ PFA Ablation System is an investigational device and is limited by Federal (or United States) Law to investigational use.



