Summary
- Kazia Therapeutics’ paxalisib awarded Rare Pediatric Disease Designation by the US FDA.
- With the RPDD grant, Kazia Therapeutics may now be eligible to receive a rare pediatric disease priority review voucher if paxalisib gets approved for Diffuse Intrinsic Pontine Glioma.
- MGC Pharmaceuticals confirms the effectiveness of ArtemiC as an immune-modulatory agent for the treatment of COVID-19.
- The Company is awaiting the first interim findings from the COVID-19 patients from the Phase 2 clinical trial underway in Israel, which are anticipated to be finalised soon.
The share price of two ASX-listed healthcare players Kazia Therapeutics Limited (ASX:KZA) and MGC Pharmaceuticals Ltd (ASX:MGC) skyrocketed today following some significant announcements.
Kazia Therapeutics Limited disclosed that its product Paxalisib was awarded as Rare Pediatric Disease Designation by the US FDA and shares of KZA soared by ~57% to A$0.880.
Another cannabis player MGC Pharmaceuticals Ltd rallied ~14% to A$0.025 after announcing effective immunological action of ArtemiC as an immune-modulator for the treating COVID-19.
Let us delve deep and discuss these announcements in detail:
US FDA Awarded Rare Pediatric Disease Designation (RPDD) to Kazia’s Paxalisib
ASX-listed innovative oncology-focused biotechnology company Kazia Therapeutics Limited (ASX:KZA) is engaged in developing therapies across a range of oncology indications. Product pipeline of Kazia comprises two clinical-stage drug development candidates paxalisib and cantrixil.
On 7 August 2020, Kazia Therapeutics announced that the US FDA had awarded Rare Pediatric Disease Designation to its drug, paxalisib (formerly known as GDC-0084) for treating a rare and highly-aggressive childhood brain cancer, Diffuse Intrinsic Pontine Glioma, or DIPG.
With the RPDD grant, Kazia may now be entitled to receive a rare pediatric disease priority review voucher if the investigational drug is approved for DIPG.
A priority review voucher (PRV) permits the holder an expedited six-month review of a new drug application by the FDA. These vouchers could be sold to other companies and have, in the past, demanded prices in the range US$68-US$350 million.
The designation has been awarded after encouraging pre-clinical results in rare childhood brain cancer and with preliminary clinical efficacy information anticipated in the second half of the calendar year 2020. The positive preliminary clinical data may substantially enhance the likelihood of a potential future priority review voucher.

Positive Clinical Data From Phase 2 study of paxalisib
On 31 July 2020, Kazia Therapeutics provided an update on the continuing development of its product candidates for the June quarter (ended 30 June 2020).
- Positive clinical data from paxalisib’s Phase 2 trial in glioblastoma was presented at the American Association for Cancer Research (AACR) and ASCO conferences in June 2020.
- Paxalisib remains on track for the potential beginning of GBM AGILE registration study in the second half of CY2020.
In addition to the current Phase 2 clinical study in glioblastoma, four trials of paxalisib in other forms of brain cancer are ongoing.
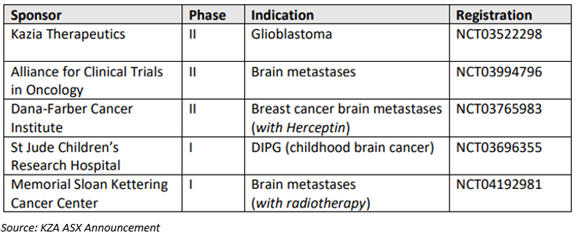
Upcoming Plans - Kazia Therapeutics’ critical milestones for the forthcoming two quarters are-
- Initiation of recruitment to GBM AGILE registration study for paxalisib in glioblastoma.
- Additional interim information from the ongoing Phase 2 clinical study of paxalisib in glioblastoma.
- The interim data from the current Phase 2 clinical trial of paxalisib in breast cancer brain metastases at Dana-Farber Breast Cancer Institute.
- The Company expects the preliminary efficacy data from the continuing Phase 1 clinical trial of paxalisib in DIPG at St Jude will be available during the second half of the CY 2020.
Moreover, the Company specified that these milestones are indicative and may be subject to change.
ALSO READ: ASX-listed Cancer Stocks Under the Microscope
Stock Performance: On 7 August 2020, KZA last quoted at A$0.880, zooming 57.143% compared to its previous close. The Company has a market cap of A$52.98 million. The stock has delivered a positive return of 47.37% in the last three months.
MGC Pharmaceuticals Confirms Effectiveness of ArtemiC as an immune-modulatory agent for COVID-19
ASX-listed European biopharma player MGC Pharmaceuticals Ltd (ASX:MXC) is engaged in development as well as the supply of affordable standardised phytocannabinoid derived medicines internationally. MGC has a strong product portfolio that offers targeting two common medical conditions epilepsy and dementia, with further products are underway.
On 7 August 2020, MGC Pharma disclosed results from pre-clinical in-vitro lab tests which have demonstrated effective immunological activity for using ArtemiC as an immune-modulator for COVID-19 treatment.
ArtemiC-In-vitro Study Findings
The findings of the study indicate that ArtemiC could decrease the risk of inflammation by reducing the interleukins (IL-1β and IL-6) release from Peripheral Blood Mononuclear Cells (PBMCs) in response to standard stimulation by exposure to Lipopolysaccharide.
Interleukins activate inflammation & modulate the immune response, and Lipopolysaccharide causes the generation of cytokines that leads to cytokines storm.
The research information suggests that the ArtemiC active ingredients combination is more efficient and elicit an in-vitro effect on immunological reaction during a disease.
MGC Pharma is presently managing pre-clinical studies in parallel to the Phase 2a clinical trial. The focus of the pre-clinical study is to demonstrate the safety, toxicity, and MOA of ArtemiC as a drug for COVID-19.
Currently, the Company is awaiting the first interim findings from the COVID-19 patients from the Phase 2 clinical trial underway in Israel, which are anticipated to be finalised shortly.

Stock Information: On 7 August 2020, MXC stock settled the day’s trade at A$0.025, climbing by 13.636%. With a market cap of nearly A$34.84 million, MXC has ~1.58 billion shares trading on the ASX.